よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (33 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_34345.html |
| 出典情報 | 厚生科学審議会 科学技術部会 全ゲノム解析等の推進に関する専門委員会(第16回 7/226)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
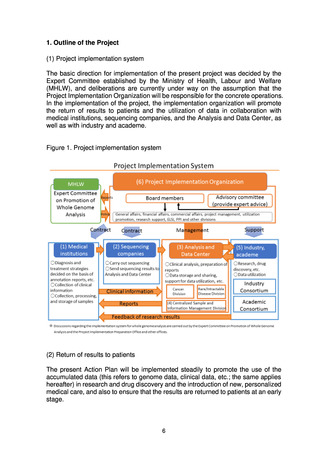
6. Policy and Details of Project Operations
(1) Return of results to patients
(a) Outline of the system for return of results to patients
To ensure that patients can receive high-quality medical care based on the
results of whole genome analysis regardless of where they live, return of
results to patients will have to be carried out through standardized methods
and also on the basis of analysis and report formats that take into account the
particular characteristics of the fields of cancer and rare/intractable diseases.
1) System A (own institution autonomous system)
In System A, clinical genome analysis7 and preparing reports are carried
out at the medical institution (or an affiliated medical institution). Specifically,
after sequencing is carried out by the sequencing company, the medical
institution (or an affiliated medical institution) conducts clinical genome
analysis of the FASTQ data that are generated and prepares a report, and
its expert panel carries out discussions. The medical institution can then
provide patients with personalized medical care.
Medical institutions under System A cooperate with each other to
improve the accuracy of their clinical genome analysis and report
preparation. In addition, the accuracy of reports prepared by the medical
institution (or an affiliated medical institution) is evaluated by the Analysis
and Data Center to guarantee the quality of reports.
7 This refers to the analysis (mapping, variant calling) and determination of clinicopathological
significance of FASTQ data generated after sequencing by a sequencing company.
32
(1) Return of results to patients
(a) Outline of the system for return of results to patients
To ensure that patients can receive high-quality medical care based on the
results of whole genome analysis regardless of where they live, return of
results to patients will have to be carried out through standardized methods
and also on the basis of analysis and report formats that take into account the
particular characteristics of the fields of cancer and rare/intractable diseases.
1) System A (own institution autonomous system)
In System A, clinical genome analysis7 and preparing reports are carried
out at the medical institution (or an affiliated medical institution). Specifically,
after sequencing is carried out by the sequencing company, the medical
institution (or an affiliated medical institution) conducts clinical genome
analysis of the FASTQ data that are generated and prepares a report, and
its expert panel carries out discussions. The medical institution can then
provide patients with personalized medical care.
Medical institutions under System A cooperate with each other to
improve the accuracy of their clinical genome analysis and report
preparation. In addition, the accuracy of reports prepared by the medical
institution (or an affiliated medical institution) is evaluated by the Analysis
and Data Center to guarantee the quality of reports.
7 This refers to the analysis (mapping, variant calling) and determination of clinicopathological
significance of FASTQ data generated after sequencing by a sequencing company.
32