よむ、つかう、まなぶ。
【資料No.1】1.12_添付資料一覧(PDF:5.91MB (16 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26901.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第3回 7/20)、医薬品第二部会(令和4年度第6回 7/20)(合同開催)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
S-217622
1.12
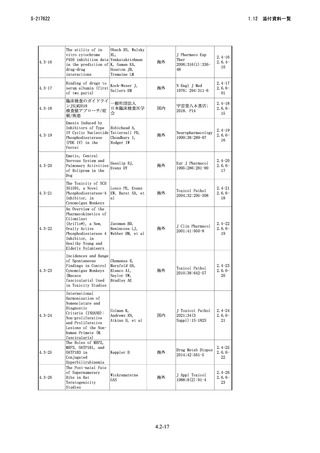
4.3-06
Borea PA,
Pharmacology of
Stefania G,
Adenosine
Stefania M,
Receptors:The State
Fabrizio V,
of the Art
Katia V
海外
4.3-07
医薬品開発と適正な情 厚生労働省医薬・
報提供のための薬物相 生活衛生局医薬品
互作用ガイドライン 審査管理課
国内
―
2.4-07
2.6.401
4.3-08
In vitro Drug
Interaction
Studies—Cytochrome
P450 Enzyme- and
Transporter-Mediated
Drug Interactions
海外
―
2.4-08
2.6.402
4.3-09
Committee for Human
Medicinal Products.
European
Guideline on the
Medicines Agency
Investigation of
Drug Interactions
海外
―
2.4-09
2.6.403
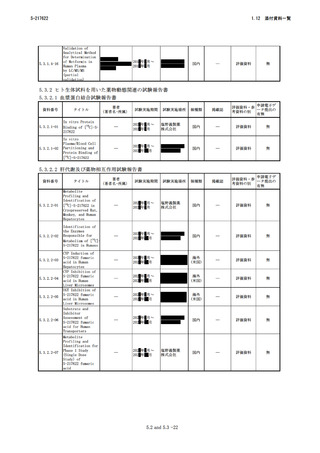
4.3-10
4.3-11
4.3-12
4.3-13
4.3-14
4.3-15
Genetic
Polymorphisms in
ADORA2A and CYP1A2
Influence
Caffeine’s Effect
on Postprandial
Glycaemia
Evaluation of CYP2B6
Induction and
Prediction of
Clinical DDI using
PBPK Modeling
Progress in
Prediction and
Interpretation of
Clinically Relevant
Metabolic Drug-Drug
Interactions: a
Minireview
Illustrating Recent
Developments and
Current
Opportunities
Estimation of
Interindividual
Variability of
Pharmacokinetics of
CYP2C9 Substrates in
Humans
(-)- N-3Benzylphenobarbital
Is Superior to
Omeprazole and (+)N-3-Benzylnirvanol
as a CYP2C19
Inhibitor in
Suspended Human
Hepatocytes
Prediction of CYP2D6
drug interactions
from in vitro data:
evidence for
substrate-dependent
inhibition
U.S. Department
of Health and
Human Services
Food and Drug
Administration
Center for Drug
Evaluation and
Research
Physiol Rev
2018;98(3):1591625
2.4-06
2.6.204
Banks NF, Tomko
PM, Colquhoun
RJ, Muddle TWD,
Emerson SR,
Jenkins NDM
海外
Sci Rep
2019;19;9(1):
10532
2.4-10
2.6.404
Ke AB, Barter Z,
Rowland-Yeo K
海外
ASCPT Annual
Meeting, March
15‐18, 2017,
Washington DC
2.4-11
2.6.405
Fowler S, Morcos
P, Cleary Y, et
al
海外
Curr Pharmacol
2.4-12
Rep 2017;3 (1):36- 2.6.449
06
Chiba K, Shimizu
K, Kato M, et al
海外
J Pharm Sci
2.4-13
2017;106(9):2695- 2.6.4703
07
Cuypers ML,
Chanteux H,
Gillent E, et al
海外
Drug Metab Dispos 2.4-14
2020;48(11):1121- 2.6.48
08
VandenBrink BM,
Foti RS, Rock
DA, Wienkers LC,
Wahlstrom JL
海外
2.4-15
Drug Metab Dispos
2.6.42012;40(1):47-53
09
4.2-16
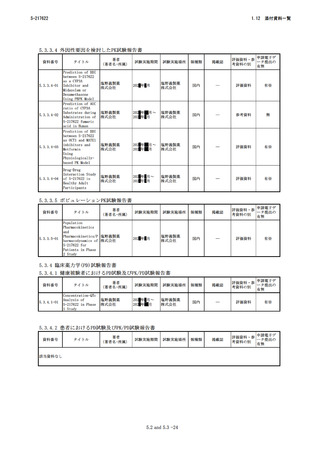
添付資料一覧
1.12
4.3-06
Borea PA,
Pharmacology of
Stefania G,
Adenosine
Stefania M,
Receptors:The State
Fabrizio V,
of the Art
Katia V
海外
4.3-07
医薬品開発と適正な情 厚生労働省医薬・
報提供のための薬物相 生活衛生局医薬品
互作用ガイドライン 審査管理課
国内
―
2.4-07
2.6.401
4.3-08
In vitro Drug
Interaction
Studies—Cytochrome
P450 Enzyme- and
Transporter-Mediated
Drug Interactions
海外
―
2.4-08
2.6.402
4.3-09
Committee for Human
Medicinal Products.
European
Guideline on the
Medicines Agency
Investigation of
Drug Interactions
海外
―
2.4-09
2.6.403
4.3-10
4.3-11
4.3-12
4.3-13
4.3-14
4.3-15
Genetic
Polymorphisms in
ADORA2A and CYP1A2
Influence
Caffeine’s Effect
on Postprandial
Glycaemia
Evaluation of CYP2B6
Induction and
Prediction of
Clinical DDI using
PBPK Modeling
Progress in
Prediction and
Interpretation of
Clinically Relevant
Metabolic Drug-Drug
Interactions: a
Minireview
Illustrating Recent
Developments and
Current
Opportunities
Estimation of
Interindividual
Variability of
Pharmacokinetics of
CYP2C9 Substrates in
Humans
(-)- N-3Benzylphenobarbital
Is Superior to
Omeprazole and (+)N-3-Benzylnirvanol
as a CYP2C19
Inhibitor in
Suspended Human
Hepatocytes
Prediction of CYP2D6
drug interactions
from in vitro data:
evidence for
substrate-dependent
inhibition
U.S. Department
of Health and
Human Services
Food and Drug
Administration
Center for Drug
Evaluation and
Research
Physiol Rev
2018;98(3):1591625
2.4-06
2.6.204
Banks NF, Tomko
PM, Colquhoun
RJ, Muddle TWD,
Emerson SR,
Jenkins NDM
海外
Sci Rep
2019;19;9(1):
10532
2.4-10
2.6.404
Ke AB, Barter Z,
Rowland-Yeo K
海外
ASCPT Annual
Meeting, March
15‐18, 2017,
Washington DC
2.4-11
2.6.405
Fowler S, Morcos
P, Cleary Y, et
al
海外
Curr Pharmacol
2.4-12
Rep 2017;3 (1):36- 2.6.449
06
Chiba K, Shimizu
K, Kato M, et al
海外
J Pharm Sci
2.4-13
2017;106(9):2695- 2.6.4703
07
Cuypers ML,
Chanteux H,
Gillent E, et al
海外
Drug Metab Dispos 2.4-14
2020;48(11):1121- 2.6.48
08
VandenBrink BM,
Foti RS, Rock
DA, Wienkers LC,
Wahlstrom JL
海外
2.4-15
Drug Metab Dispos
2.6.42012;40(1):47-53
09
4.2-16
添付資料一覧