よむ、つかう、まなぶ。
参考資料9 Action plan for whole genome analysis 2022 (26 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_31469.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第14回 3/9)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
(c) Analysis and Data Center
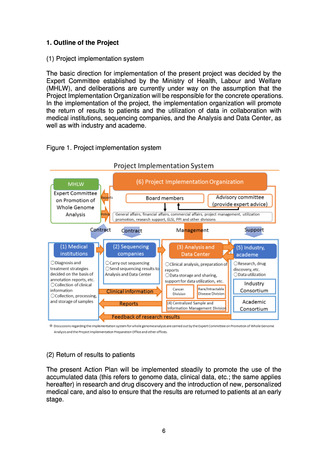
The Analysis and Data Center will store, share, and support the utilization of
sequencing results collected from sequencing companies and clinical
information collected from medical institutions. The Analysis and Data Center
is required to carry out the following four roles:
1) Analysis of genome data
2) Collection of clinical data
3) Utilization of data
4) Development of human resources
1) Analysis of genome data
o Genome database
A genome database will be constructed in the Analysis and Data Center
that can appropriately collect and store sequencing results from sequencing
companies. This will need to have systems compatible with international
research, while ensuring data security by enabling efficient data transfer via
cloud computing.
o Unified pipeline
A unified pipeline will be created and operated to carry out primary analysis,
from FASTQ files to the creation of VCF files, by means of a unified method.
o Report preparation system
A system will be established to prepare reports that are easy for both
physicians and patients to understand, which will include the clinical
significance after primary analysis and clinical trial information that reflects
the clinical information of individual patients, etc.
o Advanced cross-sectional analysis
In cooperation with industry and academe, the Analysis and Data Center
will use the collected genomic data and clinical information to conduct
advanced cross-sectional analysis by domain or across domains to
establish systems able to promptly return new findings to patients.
2) Collection of clinical data
o Clinical data collection system
A system will be created within the Analysis and Data Center for
management of clinical information collected from multiple medical
institutions in a form that will allow comparison.
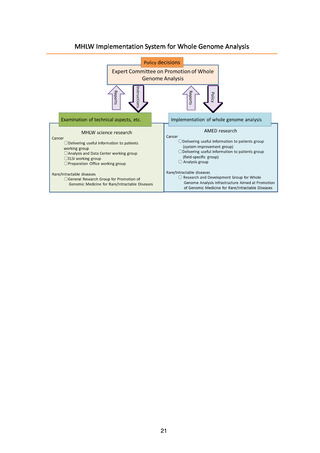
During FY2022, the AMED research group will take the lead in
examining the standardization of clinical information at multiple medical
institutions and the establishment of a clinical information collection system.
Following the launch of the Project Implementation Organization, the
aim will be to construct a system that extracts necessary data from
electronic medical records using an application programming interface
(API), with no need to manually transcribe and input data into the system,
so that the Analysis and Data Center can access data directly.
3) Utilization of data
25
The Analysis and Data Center will store, share, and support the utilization of
sequencing results collected from sequencing companies and clinical
information collected from medical institutions. The Analysis and Data Center
is required to carry out the following four roles:
1) Analysis of genome data
2) Collection of clinical data
3) Utilization of data
4) Development of human resources
1) Analysis of genome data
o Genome database
A genome database will be constructed in the Analysis and Data Center
that can appropriately collect and store sequencing results from sequencing
companies. This will need to have systems compatible with international
research, while ensuring data security by enabling efficient data transfer via
cloud computing.
o Unified pipeline
A unified pipeline will be created and operated to carry out primary analysis,
from FASTQ files to the creation of VCF files, by means of a unified method.
o Report preparation system
A system will be established to prepare reports that are easy for both
physicians and patients to understand, which will include the clinical
significance after primary analysis and clinical trial information that reflects
the clinical information of individual patients, etc.
o Advanced cross-sectional analysis
In cooperation with industry and academe, the Analysis and Data Center
will use the collected genomic data and clinical information to conduct
advanced cross-sectional analysis by domain or across domains to
establish systems able to promptly return new findings to patients.
2) Collection of clinical data
o Clinical data collection system
A system will be created within the Analysis and Data Center for
management of clinical information collected from multiple medical
institutions in a form that will allow comparison.
During FY2022, the AMED research group will take the lead in
examining the standardization of clinical information at multiple medical
institutions and the establishment of a clinical information collection system.
Following the launch of the Project Implementation Organization, the
aim will be to construct a system that extracts necessary data from
electronic medical records using an application programming interface
(API), with no need to manually transcribe and input data into the system,
so that the Analysis and Data Center can access data directly.
3) Utilization of data
25