よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (16 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_33324.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第15回 5/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
4. Initiatives to Date
Cancer
In the field of cancer, the Action Plan (Version 1) stipulates that, “First, the
characteristics of mutations in the genome of Japanese people will be clarified by
preliminary analysis, in order to advance policy decisions and development of
systems for full-scale analysis.” With regard to the preliminary analysis, the Action
Plan (Version 1) stipulates that, “For the present, samples will be selected for use
that meet the conditions, such as whether the patient’s consent has been obtained
for the use of analysis results, whether the stored samples are of sufficient quality
for analysis, and whether clinical information is available. Taking into account the
opinion of an advisory council, whole genome analysis of refractory cancers with
relatively low 5-year survival rates, rare cancers (including pediatric cancers) that
are often caused by rare genetic alterations, and hereditary cancers (including
pediatric cancers) will be conducted, to the extent possible with the current human
resources and facilities.”
On this basis, whole genome analysis was conducted on previously stored
samples from 550 cases of refractory cancer and 3,247 cases of hereditary cancer
between FY2019 and FY2020. The preliminary analyses1 included verification of
technical issues when performing whole genome analysis,2 creation of a shared
platform (ensuring data of sufficient quality in sequences obtained using a single
sequencer), and creation of a unified analysis pipeline (from FASTQ file to mutation
calls). Furthermore, results such as the identification of pathological mutations that
could not be detected by conventional genetic testing methods3 demonstrated the
significance of whole genome analysis.
At the same time, issues were identified. These included the establishment of
a pipeline for structural aberrations, which are results that whole genome analysis
is particularly expected to deliver, and splicing mutations that require integrated
analysis with transcriptome analysis; speeding up the process of determining the
clinical significance of whole genome analysis results; establishment of an expert
panel to handle whole genome analysis results; and creation of a system for
verification and analysis of whole genome analysis results.
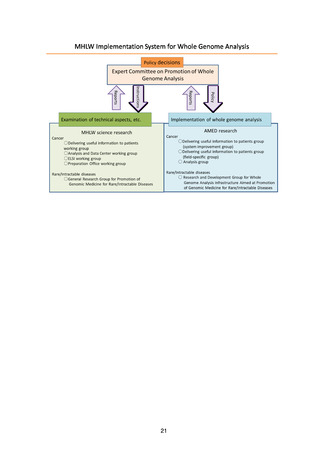
In addition, the Liaison and Coordination Council for Whole Genome Analysis of
Cancer (tentative name) and the Study Council for Promotion of the Action Plan for
Whole Genome Analysis (tentative name) held discussions on policy for the fullscale analysis and system development, and the results of these discussions are
1
Practical Research for Innovative Cancer Control Research Group (Research representative:
Teruhiko Yoshida, National Cancer Center Hospital), MHLW funded Comprehensive Research
Project for the Promotion of Cancer Countermeasures Research Group (Research representative:
Noboru Yamamoto, National Cancer Center Hospital)
2 Evaluation of sequencing depth of tumor and normal areas in whole genome analysis, integrated
analysis with transcriptome analysis, cross-tissue analysis of somatic mutations, global genome
mutation analysis (including driver gene frequency analysis compared to Europe and the U.S.),
chronological sample analysis, etc.
3 For example, in cases in which the possibility of hereditary tumor was clinically suspected, but
there was no definitive diagnosis, whole genome analysis detected a deep intronic mutation in the
ATM gene (which encodes a protein involved in cell cycle control and DNA repair and is thought to
be involved in the development of breast and ovarian cancer), and a splicing abnormality was
confirmed by combined RNA sequencing. In addition, whole genome analysis was able to identify
mutations characteristic of breast cancer, bone and soft tissue tumors, pancreatic cancer, and
leukemia.
15
Cancer
In the field of cancer, the Action Plan (Version 1) stipulates that, “First, the
characteristics of mutations in the genome of Japanese people will be clarified by
preliminary analysis, in order to advance policy decisions and development of
systems for full-scale analysis.” With regard to the preliminary analysis, the Action
Plan (Version 1) stipulates that, “For the present, samples will be selected for use
that meet the conditions, such as whether the patient’s consent has been obtained
for the use of analysis results, whether the stored samples are of sufficient quality
for analysis, and whether clinical information is available. Taking into account the
opinion of an advisory council, whole genome analysis of refractory cancers with
relatively low 5-year survival rates, rare cancers (including pediatric cancers) that
are often caused by rare genetic alterations, and hereditary cancers (including
pediatric cancers) will be conducted, to the extent possible with the current human
resources and facilities.”
On this basis, whole genome analysis was conducted on previously stored
samples from 550 cases of refractory cancer and 3,247 cases of hereditary cancer
between FY2019 and FY2020. The preliminary analyses1 included verification of
technical issues when performing whole genome analysis,2 creation of a shared
platform (ensuring data of sufficient quality in sequences obtained using a single
sequencer), and creation of a unified analysis pipeline (from FASTQ file to mutation
calls). Furthermore, results such as the identification of pathological mutations that
could not be detected by conventional genetic testing methods3 demonstrated the
significance of whole genome analysis.
At the same time, issues were identified. These included the establishment of
a pipeline for structural aberrations, which are results that whole genome analysis
is particularly expected to deliver, and splicing mutations that require integrated
analysis with transcriptome analysis; speeding up the process of determining the
clinical significance of whole genome analysis results; establishment of an expert
panel to handle whole genome analysis results; and creation of a system for
verification and analysis of whole genome analysis results.
In addition, the Liaison and Coordination Council for Whole Genome Analysis of
Cancer (tentative name) and the Study Council for Promotion of the Action Plan for
Whole Genome Analysis (tentative name) held discussions on policy for the fullscale analysis and system development, and the results of these discussions are
1
Practical Research for Innovative Cancer Control Research Group (Research representative:
Teruhiko Yoshida, National Cancer Center Hospital), MHLW funded Comprehensive Research
Project for the Promotion of Cancer Countermeasures Research Group (Research representative:
Noboru Yamamoto, National Cancer Center Hospital)
2 Evaluation of sequencing depth of tumor and normal areas in whole genome analysis, integrated
analysis with transcriptome analysis, cross-tissue analysis of somatic mutations, global genome
mutation analysis (including driver gene frequency analysis compared to Europe and the U.S.),
chronological sample analysis, etc.
3 For example, in cases in which the possibility of hereditary tumor was clinically suspected, but
there was no definitive diagnosis, whole genome analysis detected a deep intronic mutation in the
ATM gene (which encodes a protein involved in cell cycle control and DNA repair and is thought to
be involved in the development of breast and ovarian cancer), and a splicing abnormality was
confirmed by combined RNA sequencing. In addition, whole genome analysis was able to identify
mutations characteristic of breast cancer, bone and soft tissue tumors, pancreatic cancer, and
leukemia.
15