よむ、つかう、まなぶ。
慶應義塾大学 岸本特任教授 御提出資料 (3 ページ)
出典
| 公開元URL | https://www8.cao.go.jp/kisei-kaikaku/kisei/meeting/wg/2310_04medical/231218/medical04_agenda.html |
| 出典情報 | 規制改革推進会議 健康・医療・介護ワーキング・グループ(第4回 12/18)《内閣府》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Psychiatry and
Clinical Neurosciences
among the groups regarding types of disorders, randomization was
performed by a blinded, independent third party using a modified
minimization method and biased-coin assignment22 balanced for age
group (≥60 years or <60 years), sex (male or female), target disorder,
and participating institution. Additionally, the allocation results were
not disclosed to the central evaluator to minimize bias.
Assessment Schedule
After randomization, participants completed the following assessments
through self-rating scales and interviews as baseline assessments and
again at weeks 12 and 24.
Primary Outcome
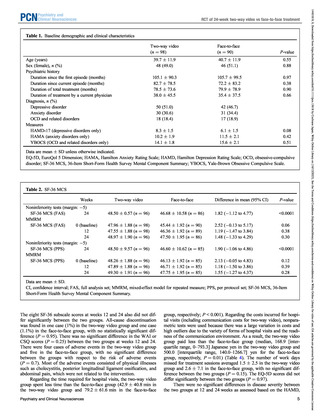
The primary outcome was the 36-Item Short-Form Health Survey
Mental Component Summary (SF-36 MCS) score at week 24. The
SF-36, a scientifically validated and reliable instrument for assessing
health-related quality of life, consists of a self-administered questionnaire23 to which patients in this study responded through a dedicated
application. The SF-36 MCS focuses on mental items and was used
because the present study targets multiple psychiatric disorders.
Secondary Outcomes
The following secondary outcomes were assessed: (1) SF-36 Physical
Component Summary (PCS) scores; (2) all-cause discontinuation
(in the two-way video group, if the patient discontinued two-way video
and switched to face-to-face treatment only, the patient was considered
to have dropped out of the two-way video group; (3) Working Alliance
Inventory (WAI) score (assessed at weeks 12 and 24) as a measure of
treatment alliance24; (4) Client Satisfaction Questionnaire (CSQ) score
(assessed at weeks 12 and 24) for assessing satisfaction25; (5) adverse
events; (6) cost and time (assessed using a self-administered questionnaire on costs and time associated with medical treatments);
(7) EuroQol 5 Dimension (EQ-5D) score (assessed at baseline and at
weeks 12 and 24) as another measure of health-related quality of life26;
(8) degree of anxiety regarding coronavirus disease 2019 (COVID-19);
(9) comments about two-way video; (10) for the depressive disorder
group, the Hamilton Depression Rating Scale (HAMD) score27;
(11) for the anxiety disorder group, the Hamilton Anxiety Rating Scale
(HAMA) score28; and (12) for the OCD and related disorders group,
the Yale-Brown Obsessive Compulsive Scale (YBOCS) score.29
Sample Size
The sample size was calculated based on previous psychiatric intervention studies (including those involving psychotherapy and electroconvulsive therapy interventions), in which the evaluation period was
6 months.30–35
In previous studies, the mean SF-36 MCS scores ranged from
30 to 50 (SD, 9–14). In the present study, assuming that an SF-36
MCS score of 45 in both the two-way video and face-to-face groups at
6 months (no difference between the two groups), with an SD of
12 and a noninferiority margin of five, the required number of patients
in each group would be 92 under the conditions of 80% power and a
one-sided significance level of 2.5%.
The all-cause discontinuation rate was expected to be low in this
study, because the primary psychiatrist who had been treating a
patient until the time of the study would continue to be in charge of
the treatment, regardless of whether the patient was in the two-way
video or face-to-face group. Assuming an all-cause discontinuation
rate of approximately 10%, the total number of required patients was
calculated as 200, or 100 in each group.
Data Collection and Management
Data on the SF-36 MCS and SF-36 PCS scores, treatment alliance and
satisfaction measures, cost, EQ-5D score, and degree of anxiety about
COVID-19 were collected as self-administered patient-reported values.
All such electronic patient-reported outcome data were collected through
the participants’ smartphones using an electronic data capture system.
Psychiatry and Clinical Neurosciences
RCT of 24-week two-way video vs face-to-face treatment
For the HAMD, HAMA, and YBOCS scores, remote centralized ratings
were obtained through two-way video. Evaluators were required to have
completed a total of at least 30 h of training on these evaluation items.
Statistical Analyses
The full analysis set (FAS), which included all patients who completed
at least one SF-36 MCS assessment during the study period and did
not present any serious violation of the study protocol or the ethical
research guidelines, was used for the analysis of the primary outcome.
The per-protocol set (PPS), which is the supplemental analysis population for the primary outcome, was defined as the population excluding
patients in serious violation of the study protocol from among the
FAS, i.e. (1) violation of selection/exclusion criteria; (2) violation of
discontinuation criteria; (3) violations related to therapies for which
concomitant use was prohibited; and (4) lack of follow-up data. The
primary analysis was performed for FAS and PPS, and secondary efficacy analyses and exploratory analyses were conducted only for FAS.
Safety analysis was performed on the safety analysis set, which was
defined as the set of patients enrolled in the study and who underwent
at least one SF-36 MCS assessment in addition to that at baseline. As
appropriate, χ2 and Fisher exact tests were used for categorical variables, while Wilcoxon rank sum test and t test were employed for continuous variables. In the primary analysis, point estimates and their
95% confidence intervals were estimated for each time point using a
mixed-effects model for repeated measures. The correlation structure
was assumed to be unstructured. Adjustment factors for allocation were
adjusted, a restricted maximum likelihood estimator was used as the
estimator of each parameter, and the Kenward-Roger method was used
to estimate the variance of the parameter estimators and the degrees of
freedom.36 The noninferiority margin was set to 5. The statistical
analysis plan was developed by the principal investigator and the biostatistician before the completion of patient recruitment and data fixation. A one-sided P-value <0.025 and a two-sided P-value <0.05 were
considered statistically significant. Statistical analyses were performed
with SAS version 9.4 (SAS Institute Inc.).
In addition to the analyses described above, the possibility was
considered that there might have been a difference in the efficacy of
the treatment in the two-way video group that used as many telemedicine visits as possible versus the group that did not. Therefore, as a
post hoc analysis, we performed the same analysis for the primary
end point in the patients’ group that had 100% of their postbaseline
visits performed via two-way video.
Ethical Considerations
This study was approved by the institutional review board of the
National Center of Neurology and Psychiatry and the participating
medical facilities. The trial was registered with the Japan Registry of
Clinical Trials (jRCT1030210037). Written informed consent was
obtained from all participants. The study procedures were conducted
according to the Declaration of Helsinki.
Results
A total of 199 patients were assessed for eligibility, provided consent
to participate in the study, and were randomized into either the twoway video or face-to-face group. One hundred five patients were allocated to the two-way video group (53 with a depressive disorder,
34 with an anxiety disorder, and 18 with OCD) and 94 patients were
allocated to the face-to-face group (45 with a depressive disorder,
32 with an anxiety disorder, and 17 with OCD). Seven patients in the
two-way video group discontinued intervention due to the following
reasons: withdrawal of consent (n = 1), failure to meet the inclusion
criteria (n = 1), adverse event (n = 1), patient request (n = 1), time
commitment challenges (n = 1), and other reasons (n = 2). Four
patients in the face-to-face group discontinued intervention due to the
following reasons: loss to follow-up (n = 3) and withdrawal of consent (n = 1). The CONSORT (Consolidated Standards of Reporting
Trials) diagram for this study is presented in the Fig. 1
3
14401819, 0, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/pcn.13618 by Cochrane Japan, Wiley Online Library on [16/12/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
PCN
Clinical Neurosciences
among the groups regarding types of disorders, randomization was
performed by a blinded, independent third party using a modified
minimization method and biased-coin assignment22 balanced for age
group (≥60 years or <60 years), sex (male or female), target disorder,
and participating institution. Additionally, the allocation results were
not disclosed to the central evaluator to minimize bias.
Assessment Schedule
After randomization, participants completed the following assessments
through self-rating scales and interviews as baseline assessments and
again at weeks 12 and 24.
Primary Outcome
The primary outcome was the 36-Item Short-Form Health Survey
Mental Component Summary (SF-36 MCS) score at week 24. The
SF-36, a scientifically validated and reliable instrument for assessing
health-related quality of life, consists of a self-administered questionnaire23 to which patients in this study responded through a dedicated
application. The SF-36 MCS focuses on mental items and was used
because the present study targets multiple psychiatric disorders.
Secondary Outcomes
The following secondary outcomes were assessed: (1) SF-36 Physical
Component Summary (PCS) scores; (2) all-cause discontinuation
(in the two-way video group, if the patient discontinued two-way video
and switched to face-to-face treatment only, the patient was considered
to have dropped out of the two-way video group; (3) Working Alliance
Inventory (WAI) score (assessed at weeks 12 and 24) as a measure of
treatment alliance24; (4) Client Satisfaction Questionnaire (CSQ) score
(assessed at weeks 12 and 24) for assessing satisfaction25; (5) adverse
events; (6) cost and time (assessed using a self-administered questionnaire on costs and time associated with medical treatments);
(7) EuroQol 5 Dimension (EQ-5D) score (assessed at baseline and at
weeks 12 and 24) as another measure of health-related quality of life26;
(8) degree of anxiety regarding coronavirus disease 2019 (COVID-19);
(9) comments about two-way video; (10) for the depressive disorder
group, the Hamilton Depression Rating Scale (HAMD) score27;
(11) for the anxiety disorder group, the Hamilton Anxiety Rating Scale
(HAMA) score28; and (12) for the OCD and related disorders group,
the Yale-Brown Obsessive Compulsive Scale (YBOCS) score.29
Sample Size
The sample size was calculated based on previous psychiatric intervention studies (including those involving psychotherapy and electroconvulsive therapy interventions), in which the evaluation period was
6 months.30–35
In previous studies, the mean SF-36 MCS scores ranged from
30 to 50 (SD, 9–14). In the present study, assuming that an SF-36
MCS score of 45 in both the two-way video and face-to-face groups at
6 months (no difference between the two groups), with an SD of
12 and a noninferiority margin of five, the required number of patients
in each group would be 92 under the conditions of 80% power and a
one-sided significance level of 2.5%.
The all-cause discontinuation rate was expected to be low in this
study, because the primary psychiatrist who had been treating a
patient until the time of the study would continue to be in charge of
the treatment, regardless of whether the patient was in the two-way
video or face-to-face group. Assuming an all-cause discontinuation
rate of approximately 10%, the total number of required patients was
calculated as 200, or 100 in each group.
Data Collection and Management
Data on the SF-36 MCS and SF-36 PCS scores, treatment alliance and
satisfaction measures, cost, EQ-5D score, and degree of anxiety about
COVID-19 were collected as self-administered patient-reported values.
All such electronic patient-reported outcome data were collected through
the participants’ smartphones using an electronic data capture system.
Psychiatry and Clinical Neurosciences
RCT of 24-week two-way video vs face-to-face treatment
For the HAMD, HAMA, and YBOCS scores, remote centralized ratings
were obtained through two-way video. Evaluators were required to have
completed a total of at least 30 h of training on these evaluation items.
Statistical Analyses
The full analysis set (FAS), which included all patients who completed
at least one SF-36 MCS assessment during the study period and did
not present any serious violation of the study protocol or the ethical
research guidelines, was used for the analysis of the primary outcome.
The per-protocol set (PPS), which is the supplemental analysis population for the primary outcome, was defined as the population excluding
patients in serious violation of the study protocol from among the
FAS, i.e. (1) violation of selection/exclusion criteria; (2) violation of
discontinuation criteria; (3) violations related to therapies for which
concomitant use was prohibited; and (4) lack of follow-up data. The
primary analysis was performed for FAS and PPS, and secondary efficacy analyses and exploratory analyses were conducted only for FAS.
Safety analysis was performed on the safety analysis set, which was
defined as the set of patients enrolled in the study and who underwent
at least one SF-36 MCS assessment in addition to that at baseline. As
appropriate, χ2 and Fisher exact tests were used for categorical variables, while Wilcoxon rank sum test and t test were employed for continuous variables. In the primary analysis, point estimates and their
95% confidence intervals were estimated for each time point using a
mixed-effects model for repeated measures. The correlation structure
was assumed to be unstructured. Adjustment factors for allocation were
adjusted, a restricted maximum likelihood estimator was used as the
estimator of each parameter, and the Kenward-Roger method was used
to estimate the variance of the parameter estimators and the degrees of
freedom.36 The noninferiority margin was set to 5. The statistical
analysis plan was developed by the principal investigator and the biostatistician before the completion of patient recruitment and data fixation. A one-sided P-value <0.025 and a two-sided P-value <0.05 were
considered statistically significant. Statistical analyses were performed
with SAS version 9.4 (SAS Institute Inc.).
In addition to the analyses described above, the possibility was
considered that there might have been a difference in the efficacy of
the treatment in the two-way video group that used as many telemedicine visits as possible versus the group that did not. Therefore, as a
post hoc analysis, we performed the same analysis for the primary
end point in the patients’ group that had 100% of their postbaseline
visits performed via two-way video.
Ethical Considerations
This study was approved by the institutional review board of the
National Center of Neurology and Psychiatry and the participating
medical facilities. The trial was registered with the Japan Registry of
Clinical Trials (jRCT1030210037). Written informed consent was
obtained from all participants. The study procedures were conducted
according to the Declaration of Helsinki.
Results
A total of 199 patients were assessed for eligibility, provided consent
to participate in the study, and were randomized into either the twoway video or face-to-face group. One hundred five patients were allocated to the two-way video group (53 with a depressive disorder,
34 with an anxiety disorder, and 18 with OCD) and 94 patients were
allocated to the face-to-face group (45 with a depressive disorder,
32 with an anxiety disorder, and 17 with OCD). Seven patients in the
two-way video group discontinued intervention due to the following
reasons: withdrawal of consent (n = 1), failure to meet the inclusion
criteria (n = 1), adverse event (n = 1), patient request (n = 1), time
commitment challenges (n = 1), and other reasons (n = 2). Four
patients in the face-to-face group discontinued intervention due to the
following reasons: loss to follow-up (n = 3) and withdrawal of consent (n = 1). The CONSORT (Consolidated Standards of Reporting
Trials) diagram for this study is presented in the Fig. 1
3
14401819, 0, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/pcn.13618 by Cochrane Japan, Wiley Online Library on [16/12/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
PCN