よむ、つかう、まなぶ。
参考資料15 COVID-19 vaccine safety update; Primary series in young children and booster doses in older children and adults, ACIP September 1, 2022 (15 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00052.html |
| 出典情報 | 第85回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第14回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(10/7)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
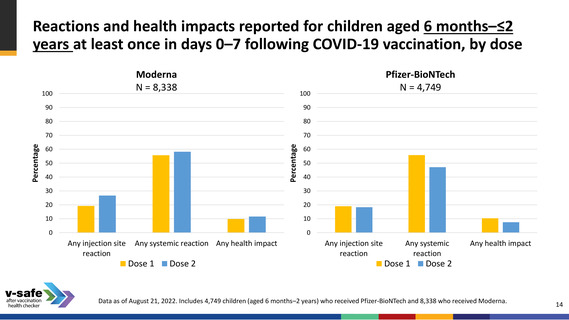
Reactions and health impacts reported for children aged 3–5 years at
least once in days 0–7 following COVID-19 vaccination, by dose
Moderna
N = 6,387
100
90
90
80
80
70
70
Percentage
Percentage
100
60
50
40
60
50
40
30
30
20
20
10
10
0
0
Any injection site
reaction
Any systemic reaction Any health impact
Dose 1
Dose 2
Pfizer-BioNTech
N = 3,792
Any injection site
reaction
Any systemic
reaction
Dose 1
Any health impact
Dose 2
Data as of August 21, 2022. Includes 3,792 children (aged 3–4 years) who received Pfizer-BioNTech and 6,387 who received Moderna (aged 3–5 years) .
15
least once in days 0–7 following COVID-19 vaccination, by dose
Moderna
N = 6,387
100
90
90
80
80
70
70
Percentage
Percentage
100
60
50
40
60
50
40
30
30
20
20
10
10
0
0
Any injection site
reaction
Any systemic reaction Any health impact
Dose 1
Dose 2
Pfizer-BioNTech
N = 3,792
Any injection site
reaction
Any systemic
reaction
Dose 1
Any health impact
Dose 2
Data as of August 21, 2022. Includes 3,792 children (aged 3–4 years) who received Pfizer-BioNTech and 6,387 who received Moderna (aged 3–5 years) .
15