よむ、つかう、まなぶ。
参考資料15 COVID-19 vaccine safety update; Primary series in young children and booster doses in older children and adults, ACIP September 1, 2022 (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00052.html |
| 出典情報 | 第85回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第14回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(10/7)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
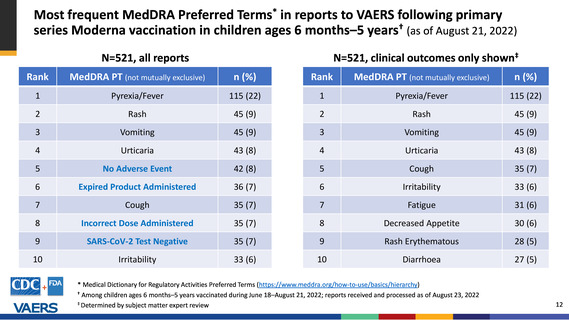
Most frequent MedDRA Preferred Terms* in reports to VAERS following 2nd booster dose
mRNA COVID-19 vaccinations, ages ≥50 years† (as of August 21, 2022)
N=11,895, non-serious reports (clinical outcomes)
N=724, serious reports (clinical outcomes)
Rank
Adverse event (not mutually exclusive)
n (%)
Rank
Adverse event (not mutually exclusive)
n (%)
1
COVID-19
3,951 (33)
1
COVID-19
218 (30)
2
SARS-CoV-2 Test Positive
2,757 (23)
2
SARS-CoV-2 Test Positive
165 (23)
3
Fatigue
2,057 (17)
3
Dyspnoea
91 (13)
4
Cough
1,724 (14)
4
Asthenia
76 (11)
5
Headache
1,645 (14)
5
Fatigue
76 (11)
6
Pyrexia/Fever
1,622 (14)
6
Death
66 (9)
7
Pain
1,247 (10)
7
Vaccine Breakthrough Infection
65 (9)
8
Oropharyngeal Pain
1,235 (10)
8
Headache
64 (9)
9
Rhinorrhoea
838 (7)
9
Cough
60 (8)
10
Malaise
811 (7)
10
Pyrexia/Fever
60 (8)
* Medical Dictionary for Regulatory Activities Preferred Terms (https://www.meddra.org/how-to-use/basics/hierarchy)
†
Among persons receiving Pfizer-BioNTech or Moderna dose 4 during March 29–August 21, 2022
23
mRNA COVID-19 vaccinations, ages ≥50 years† (as of August 21, 2022)
N=11,895, non-serious reports (clinical outcomes)
N=724, serious reports (clinical outcomes)
Rank
Adverse event (not mutually exclusive)
n (%)
Rank
Adverse event (not mutually exclusive)
n (%)
1
COVID-19
3,951 (33)
1
COVID-19
218 (30)
2
SARS-CoV-2 Test Positive
2,757 (23)
2
SARS-CoV-2 Test Positive
165 (23)
3
Fatigue
2,057 (17)
3
Dyspnoea
91 (13)
4
Cough
1,724 (14)
4
Asthenia
76 (11)
5
Headache
1,645 (14)
5
Fatigue
76 (11)
6
Pyrexia/Fever
1,622 (14)
6
Death
66 (9)
7
Pain
1,247 (10)
7
Vaccine Breakthrough Infection
65 (9)
8
Oropharyngeal Pain
1,235 (10)
8
Headache
64 (9)
9
Rhinorrhoea
838 (7)
9
Cough
60 (8)
10
Malaise
811 (7)
10
Pyrexia/Fever
60 (8)
* Medical Dictionary for Regulatory Activities Preferred Terms (https://www.meddra.org/how-to-use/basics/hierarchy)
†
Among persons receiving Pfizer-BioNTech or Moderna dose 4 during March 29–August 21, 2022
23