よむ、つかう、まなぶ。
参考資料15 COVID-19 vaccine safety update; Primary series in young children and booster doses in older children and adults, ACIP September 1, 2022 (34 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00052.html |
| 出典情報 | 第85回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第14回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(10/7)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
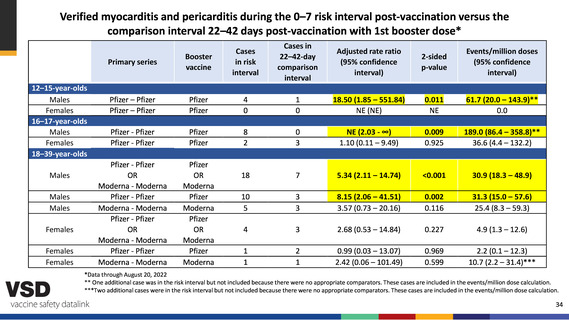
Verified myocarditis and pericarditis during the 0–7 risk interval post-vaccination versus the
comparison interval 22–42 days post-vaccination with 1st booster dose*
12–15-year-olds
Males
Females
16–17-year-olds
Males
Females
18–39-year-olds
Males
Males
Males
Females
Females
Females
Primary series
Booster
vaccine
Cases
in risk
interval
Cases in
22–42-day
comparison
interval
Adjusted rate ratio
(95% confidence
interval)
2-sided
p-value
Events/million doses
(95% confidence
interval)
Pfizer – Pfizer
Pfizer – Pfizer
Pfizer
Pfizer
4
0
1
0
18.50 (1.85 – 551.84)
NE (NE)
0.011
NE
61.7 (20.0 – 143.9)**
0.0
Pfizer - Pfizer
Pfizer - Pfizer
Pfizer
Pfizer
8
2
0
3
NE (2.03 - ∞)
1.10 (0.11 – 9.49)
0.009
0.925
189.0 (86.4 – 358.8)**
36.6 (4.4 – 132.2)
Pfizer - Pfizer
OR
Moderna - Moderna
Pfizer - Pfizer
Moderna - Moderna
Pfizer - Pfizer
OR
Moderna - Moderna
Pfizer - Pfizer
Moderna - Moderna
Pfizer
OR
Moderna
Pfizer
Moderna
Pfizer
OR
Moderna
Pfizer
Moderna
18
7
5.34 (2.11 – 14.74)
<0.001
30.9 (18.3 – 48.9)
10
5
3
3
8.15 (2.06 – 41.51)
3.57 (0.73 – 20.16)
0.002
0.116
31.3 (15.0 – 57.6)
25.4 (8.3 – 59.3)
4
3
2.68 (0.53 – 14.84)
0.227
4.9 (1.3 – 12.6)
1
1
2
1
0.99 (0.03 – 13.07)
2.42 (0.06 – 101.49)
0.969
0.599
2.2 (0.1 – 12.3)
10.7 (2.2 – 31.4)***
*Data through August 20, 2022
** One additional case was in the risk interval but not included because there were no appropriate comparators. These cases are included in the events/million dose calculation.
***Two additional cases were in the risk interval but not included because there were no appropriate comparators. These cases are included in the events/million dose calculation.
34
comparison interval 22–42 days post-vaccination with 1st booster dose*
12–15-year-olds
Males
Females
16–17-year-olds
Males
Females
18–39-year-olds
Males
Males
Males
Females
Females
Females
Primary series
Booster
vaccine
Cases
in risk
interval
Cases in
22–42-day
comparison
interval
Adjusted rate ratio
(95% confidence
interval)
2-sided
p-value
Events/million doses
(95% confidence
interval)
Pfizer – Pfizer
Pfizer – Pfizer
Pfizer
Pfizer
4
0
1
0
18.50 (1.85 – 551.84)
NE (NE)
0.011
NE
61.7 (20.0 – 143.9)**
0.0
Pfizer - Pfizer
Pfizer - Pfizer
Pfizer
Pfizer
8
2
0
3
NE (2.03 - ∞)
1.10 (0.11 – 9.49)
0.009
0.925
189.0 (86.4 – 358.8)**
36.6 (4.4 – 132.2)
Pfizer - Pfizer
OR
Moderna - Moderna
Pfizer - Pfizer
Moderna - Moderna
Pfizer - Pfizer
OR
Moderna - Moderna
Pfizer - Pfizer
Moderna - Moderna
Pfizer
OR
Moderna
Pfizer
Moderna
Pfizer
OR
Moderna
Pfizer
Moderna
18
7
5.34 (2.11 – 14.74)
<0.001
30.9 (18.3 – 48.9)
10
5
3
3
8.15 (2.06 – 41.51)
3.57 (0.73 – 20.16)
0.002
0.116
31.3 (15.0 – 57.6)
25.4 (8.3 – 59.3)
4
3
2.68 (0.53 – 14.84)
0.227
4.9 (1.3 – 12.6)
1
1
2
1
0.99 (0.03 – 13.07)
2.42 (0.06 – 101.49)
0.969
0.599
2.2 (0.1 – 12.3)
10.7 (2.2 – 31.4)***
*Data through August 20, 2022
** One additional case was in the risk interval but not included because there were no appropriate comparators. These cases are included in the events/million dose calculation.
***Two additional cases were in the risk interval but not included because there were no appropriate comparators. These cases are included in the events/million dose calculation.
34