よむ、つかう、まなぶ。
資料1-2 調査結果報告書[943KB] (10 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_45738.html |
| 出典情報 | 薬事審議会 医薬品等安全対策部会安全対策調査会(令和6年度第9回 12/4)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
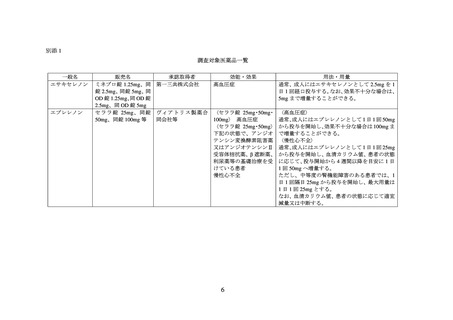
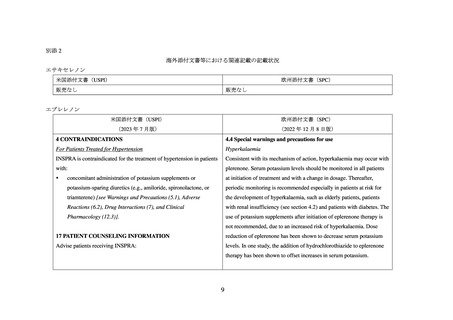
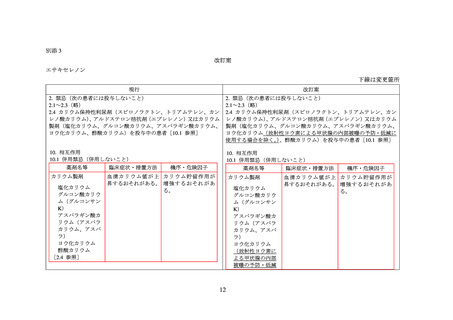
別添 2
海外添付文書等における関連記載の記載状況
エサキセレノン
米国添付文書(USPI)
欧州添付文書(SPC)
販売なし
販売なし
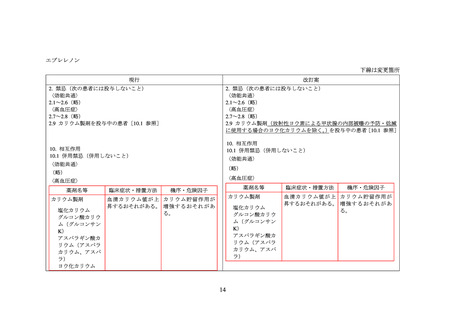
エプレレノン
米国添付文書(USPI)
欧州添付文書(SPC)
(2023 年 7 月版)
(2022 年 12 月 8 日版)
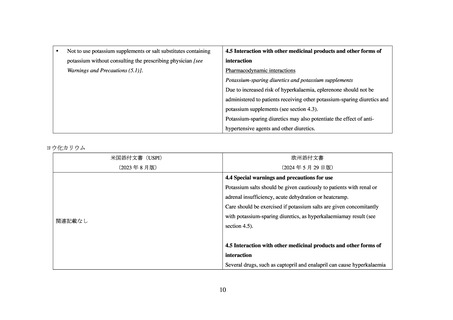
4 CONTRAINDICATIONS
4.4 Special warnings and precautions for use
For Patients Treated for Hypertension
Hyperkalaemia
INSPRA is contraindicated for the treatment of hypertension in patients
Consistent with its mechanism of action, hyperkalaemia may occur with
with:
plerenone. Serum potassium levels should be monitored in all patients
concomitant administration of potassium supplements or
at initiation of treatment and with a change in dosage. Thereafter,
potassium-sparing diuretics (e.g., amiloride, spironolactone, or
periodic monitoring is recommended especially in patients at risk for
triamterene) [see Warnings and Precautions (5.1), Adverse
the development of hyperkalaemia, such as elderly patients, patients
Reactions (6.2), Drug Interactions (7), and Clinical
with renal insufficiency (see section 4.2) and patients with diabetes. The
Pharmacology (12.3)].
use of potassium supplements after initiation of eplerenone therapy is
not recommended, due to an increased risk of hyperkalaemia. Dose
17 PATIENT COUNSELING INFORMATION
reduction of eplerenone has been shown to decrease serum potassium
Advise patients receiving INSPRA:
levels. In one study, the addition of hydrochlorothiazide to eplerenone
therapy has been shown to offset increases in serum potassium.
9
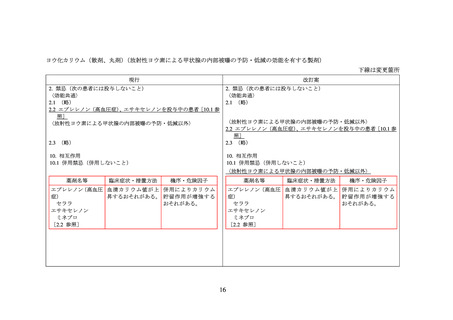
海外添付文書等における関連記載の記載状況
エサキセレノン
米国添付文書(USPI)
欧州添付文書(SPC)
販売なし
販売なし
エプレレノン
米国添付文書(USPI)
欧州添付文書(SPC)
(2023 年 7 月版)
(2022 年 12 月 8 日版)
4 CONTRAINDICATIONS
4.4 Special warnings and precautions for use
For Patients Treated for Hypertension
Hyperkalaemia
INSPRA is contraindicated for the treatment of hypertension in patients
Consistent with its mechanism of action, hyperkalaemia may occur with
with:
plerenone. Serum potassium levels should be monitored in all patients
concomitant administration of potassium supplements or
at initiation of treatment and with a change in dosage. Thereafter,
potassium-sparing diuretics (e.g., amiloride, spironolactone, or
periodic monitoring is recommended especially in patients at risk for
triamterene) [see Warnings and Precautions (5.1), Adverse
the development of hyperkalaemia, such as elderly patients, patients
Reactions (6.2), Drug Interactions (7), and Clinical
with renal insufficiency (see section 4.2) and patients with diabetes. The
Pharmacology (12.3)].
use of potassium supplements after initiation of eplerenone therapy is
not recommended, due to an increased risk of hyperkalaemia. Dose
17 PATIENT COUNSELING INFORMATION
reduction of eplerenone has been shown to decrease serum potassium
Advise patients receiving INSPRA:
levels. In one study, the addition of hydrochlorothiazide to eplerenone
therapy has been shown to offset increases in serum potassium.
9