よむ、つかう、まなぶ。
参考資料13 Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting Presentation - FDA Review of Effectiveness and Safety of Novavax COVID-19 Vaccine in Adults > 18 Years of Age - Emergency Use Authorization Request(Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting FDA提出資料) (17 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00043.html |
| 出典情報 | 第80回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第5回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(6/10)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
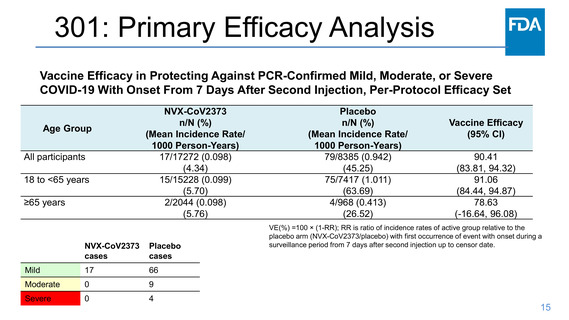
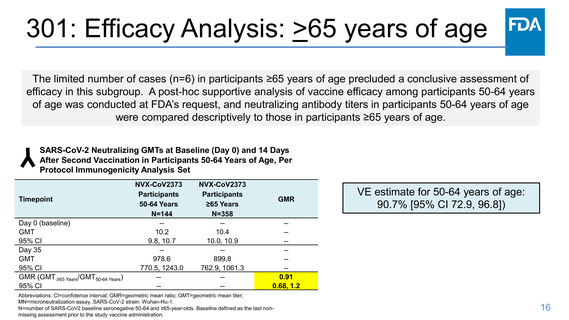
301: Efficacy Analysis: >65 years of age
The limited number of cases (n=6) in participants ≥65 years of age precluded a conclusive assessment of
efficacy in this subgroup. A post-hoc supportive analysis of vaccine efficacy among participants 50-64 years
of age was conducted at FDA’s request, and neutralizing antibody titers in participants 50-64 years of age
were compared descriptively to those in participants ≥65 years of age.
SARS-CoV-2 Neutralizing GMTs at Baseline (Day 0) and 14 Days
After Second Vaccination in Participants 50-64 Years of Age, Per
Protocol Immunogenicity Analysis Set
Timepoint
Day 0 (baseline)
GMT
95% CI
Day 35
GMT
95% CI
GMR (GMT ≥65 Years/GMT50-64 Years)
95% CI
NVX-CoV2373
Participants
50-64 Years
N=144
-10.2
9.8, 10.7
-978.6
770.5, 1243.0
---
NVX-CoV2373
Participants
≥65 Years
N=358
-10.4
10.0, 10.9
-899.8
762.9, 1061.3
---
GMR
VE estimate for 50-64 years of age:
90.7% [95% CI 72.9, 96.8])
------0.91
0.68, 1.2
Abbreviations: CI=confidence interval; GMR=geometric mean ratio; GMT=geometric mean titer;
MN=microneutralization assay, SARS-CoV-2 strain: Wuhan-Hu-1.
N=number of SARS-CoV2 baseline seronegative 50-64 and ≥65-year-olds. Baseline defined as the last nonmissing assessment prior to the study vaccine administration.
16
The limited number of cases (n=6) in participants ≥65 years of age precluded a conclusive assessment of
efficacy in this subgroup. A post-hoc supportive analysis of vaccine efficacy among participants 50-64 years
of age was conducted at FDA’s request, and neutralizing antibody titers in participants 50-64 years of age
were compared descriptively to those in participants ≥65 years of age.
SARS-CoV-2 Neutralizing GMTs at Baseline (Day 0) and 14 Days
After Second Vaccination in Participants 50-64 Years of Age, Per
Protocol Immunogenicity Analysis Set
Timepoint
Day 0 (baseline)
GMT
95% CI
Day 35
GMT
95% CI
GMR (GMT ≥65 Years/GMT50-64 Years)
95% CI
NVX-CoV2373
Participants
50-64 Years
N=144
-10.2
9.8, 10.7
-978.6
770.5, 1243.0
---
NVX-CoV2373
Participants
≥65 Years
N=358
-10.4
10.0, 10.9
-899.8
762.9, 1061.3
---
GMR
VE estimate for 50-64 years of age:
90.7% [95% CI 72.9, 96.8])
------0.91
0.68, 1.2
Abbreviations: CI=confidence interval; GMR=geometric mean ratio; GMT=geometric mean titer;
MN=microneutralization assay, SARS-CoV-2 strain: Wuhan-Hu-1.
N=number of SARS-CoV2 baseline seronegative 50-64 and ≥65-year-olds. Baseline defined as the last nonmissing assessment prior to the study vaccine administration.
16