よむ、つかう、まなぶ。
参考資料13 Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting Presentation - FDA Review of Effectiveness and Safety of Novavax COVID-19 Vaccine in Adults > 18 Years of Age - Emergency Use Authorization Request(Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting FDA提出資料) (36 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00043.html |
| 出典情報 | 第80回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第5回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(6/10)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
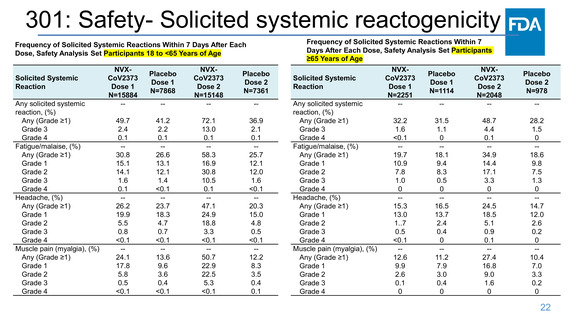
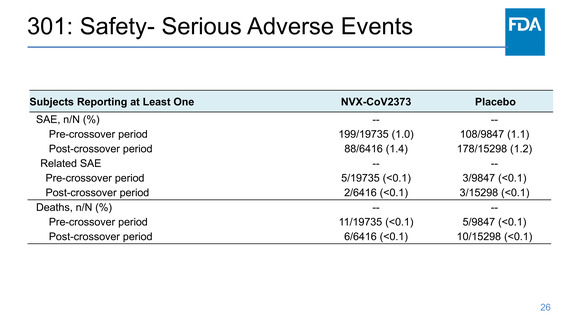
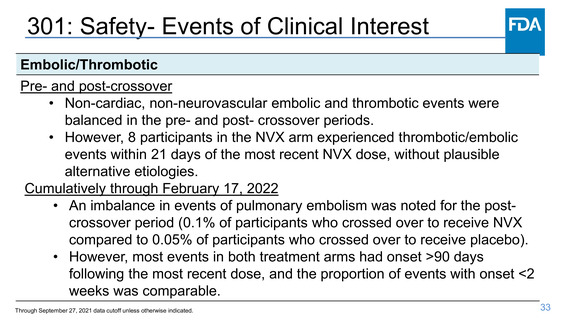
301: Safety- Events of Clinical Interest
Specific events of clinical interest/adverse events of special interest
Bell’s palsy
• In the pre-crossover period, Bell’s palsy within 30 days of vaccination was
reported by one participant each in the placebo and NVX arms.
Uveitis
• Three participants in the NVX arm had new-onset uveitis events within 3
weeks of vaccination, one of which recurred with re-challenge. Two events
of uveitis were reported in the placebo arm, one of which had onset within
one week of placebo, in a participant with a history of uveitis.
Hypersensitivity
• One event of angioedema and urticaria 2 days post-Dose 2 of NVX is
potentially related but also started concomitant antibiotic.
Through September 27, 2021 data cutoff unless otherwise indicated.
35
Specific events of clinical interest/adverse events of special interest
Bell’s palsy
• In the pre-crossover period, Bell’s palsy within 30 days of vaccination was
reported by one participant each in the placebo and NVX arms.
Uveitis
• Three participants in the NVX arm had new-onset uveitis events within 3
weeks of vaccination, one of which recurred with re-challenge. Two events
of uveitis were reported in the placebo arm, one of which had onset within
one week of placebo, in a participant with a history of uveitis.
Hypersensitivity
• One event of angioedema and urticaria 2 days post-Dose 2 of NVX is
potentially related but also started concomitant antibiotic.
Through September 27, 2021 data cutoff unless otherwise indicated.
35