よむ、つかう、まなぶ。
参考資料13 Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting Presentation - FDA Review of Effectiveness and Safety of Novavax COVID-19 Vaccine in Adults > 18 Years of Age - Emergency Use Authorization Request(Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting FDA提出資料) (42 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00043.html |
| 出典情報 | 第80回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第5回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(6/10)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
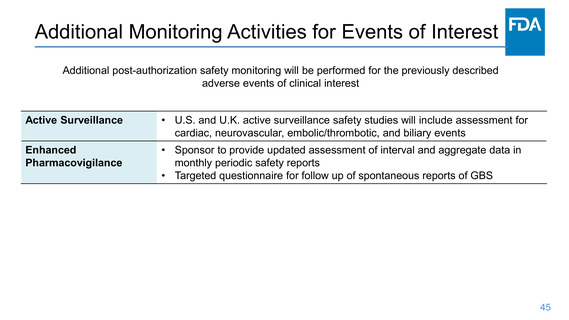
Pharmacovigilance Plan
Important identified risks
FDA recommends adding myocarditis and pericarditis
Important potential risks
Vaccine-associated enhanced disease including vaccine-associated enhanced respiratory
disease, myocarditis and pericarditis, and anaphylaxis
Missing information
Use in pregnancy and while breast feeding, use in immunocompromised patients, use in
frail patients with comorbidities (e.g., chronic obstructive pulmonary disease, diabetes,
chronic neurological disease, cardiovascular disorders), use in patients with autoimmune or
inflammatory disorders, interaction with other vaccines, and long-term safety
Surveillance activities
•
•
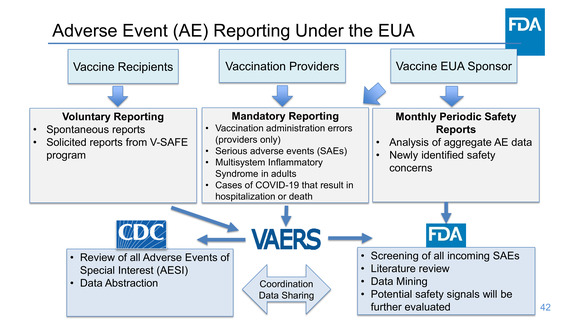
Passive surveillance activities will include submitting spontaneous reports of the
following events to the Vaccine Adverse Event Reporting System (VAERS) within 15
days: Serious adverse events (irrespective of attribution to vaccination); Cases of
Multisystem Inflammatory Syndrome in adults; Cases of COVID-19 that result in
hospitalization or death
The Sponsor will conduct:
• Passive and active surveillance activities for continued vaccine safety monitoring.
• Periodic aggregate review of safety data and submit periodic safety reports
• Five planned surveillance studies, including active follow-up studies for safety in
the US and UK
41
Important identified risks
FDA recommends adding myocarditis and pericarditis
Important potential risks
Vaccine-associated enhanced disease including vaccine-associated enhanced respiratory
disease, myocarditis and pericarditis, and anaphylaxis
Missing information
Use in pregnancy and while breast feeding, use in immunocompromised patients, use in
frail patients with comorbidities (e.g., chronic obstructive pulmonary disease, diabetes,
chronic neurological disease, cardiovascular disorders), use in patients with autoimmune or
inflammatory disorders, interaction with other vaccines, and long-term safety
Surveillance activities
•
•
Passive surveillance activities will include submitting spontaneous reports of the
following events to the Vaccine Adverse Event Reporting System (VAERS) within 15
days: Serious adverse events (irrespective of attribution to vaccination); Cases of
Multisystem Inflammatory Syndrome in adults; Cases of COVID-19 that result in
hospitalization or death
The Sponsor will conduct:
• Passive and active surveillance activities for continued vaccine safety monitoring.
• Periodic aggregate review of safety data and submit periodic safety reports
• Five planned surveillance studies, including active follow-up studies for safety in
the US and UK
41