よむ、つかう、まなぶ。
参考資料13 Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting Presentation - FDA Review of Effectiveness and Safety of Novavax COVID-19 Vaccine in Adults > 18 Years of Age - Emergency Use Authorization Request(Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting FDA提出資料) (22 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00043.html |
| 出典情報 | 第80回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第5回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(6/10)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
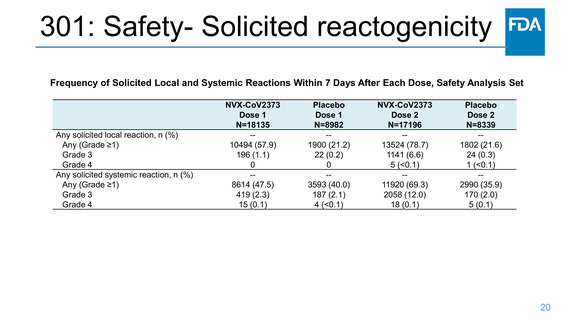
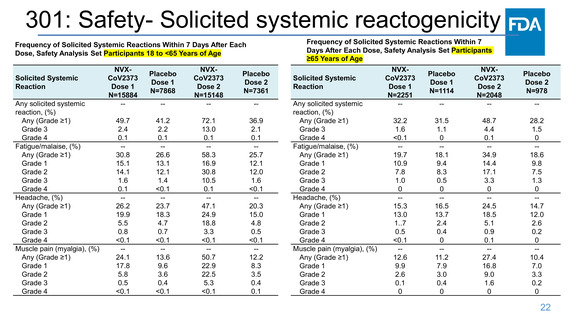
301: Safety- Solicited local reactogenicity
Frequency of Solicited Local Reactions Within 7 Days After Each
Dose, Safety Analysis Set Participants 18 to <65 Years of Age
Event
Pain/tenderness, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Erythema, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Swelling, (%)
Any (Grade ≥1)
Grade 3
Grade 4
NVXCoV2373
Dose 1
N=15884
-60.5
1.1
0
-1.0
<0.1
0
-0.9
<0.1
0
NVXPlacebo
Placebo
CoV2373
Dose 1
Dose 2
Dose 2
N=7868
N=7361
N=15148
---21.7
22.1
80.8
0.2
6.3
0.3
0
<0.1
<0.1
---0.3
0.4
6.9
0
<0.1
0.9
0
0
0
---0.3
6.2
0.3
<0.1
<0.1
0.5
0
0
0
Frequency of Solicited Local Reactions Within 7 Days After
Each Dose, Safety Analysis Set Participants ≥65 Years of Age
Event
Pain/tenderness, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Erythema, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Swelling, (%)
Any (Grade ≥1)
Grade 3
Grade 4
NVXCoV2373
Dose 1
N=2251
-37.9
0.6
0
-0.7
0
0
-0.8
<0.1
0
• Pain: Grade 1: Does not interfere with activity; Grade 3: Any use of narcotic pain reliever or prevents daily activity; and Grade 4: Emergency room
visit or hospitalization.
• Tenderness: Grade 1: Mild discomfort to touch; Grade 3: Significant discomfort at rest; and Grade 4: ER visit or hospitalization.
• Erythema: Grade 1: 2.5 to 5 cm; Grade 3: >10 cm; and Grade 4: Necrosis or exfoliative dermatitis.
• Swelling/induration: Grade 1: 2.5 to 5 cm and does not interfere with activity; Grade 3: >10 cm or prevents daily activity; and Grade 4: Necrosis.
Placebo
Dose 1
N=1114
-15.7
0.3
0
-0.5
0
0
-0.1
0
0
NVXCoV2373
Dose 2
N=2048
-61.4
2.1
0
-4.8
0.3
0
-5.4
0.4
0
Placebo
Dose 2
N=978
-16.5
0.1
0
-0.4
0
0
-0.4
0.1
0
21
Frequency of Solicited Local Reactions Within 7 Days After Each
Dose, Safety Analysis Set Participants 18 to <65 Years of Age
Event
Pain/tenderness, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Erythema, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Swelling, (%)
Any (Grade ≥1)
Grade 3
Grade 4
NVXCoV2373
Dose 1
N=15884
-60.5
1.1
0
-1.0
<0.1
0
-0.9
<0.1
0
NVXPlacebo
Placebo
CoV2373
Dose 1
Dose 2
Dose 2
N=7868
N=7361
N=15148
---21.7
22.1
80.8
0.2
6.3
0.3
0
<0.1
<0.1
---0.3
0.4
6.9
0
<0.1
0.9
0
0
0
---0.3
6.2
0.3
<0.1
<0.1
0.5
0
0
0
Frequency of Solicited Local Reactions Within 7 Days After
Each Dose, Safety Analysis Set Participants ≥65 Years of Age
Event
Pain/tenderness, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Erythema, (%)
Any (Grade ≥1)
Grade 3
Grade 4
Swelling, (%)
Any (Grade ≥1)
Grade 3
Grade 4
NVXCoV2373
Dose 1
N=2251
-37.9
0.6
0
-0.7
0
0
-0.8
<0.1
0
• Pain: Grade 1: Does not interfere with activity; Grade 3: Any use of narcotic pain reliever or prevents daily activity; and Grade 4: Emergency room
visit or hospitalization.
• Tenderness: Grade 1: Mild discomfort to touch; Grade 3: Significant discomfort at rest; and Grade 4: ER visit or hospitalization.
• Erythema: Grade 1: 2.5 to 5 cm; Grade 3: >10 cm; and Grade 4: Necrosis or exfoliative dermatitis.
• Swelling/induration: Grade 1: 2.5 to 5 cm and does not interfere with activity; Grade 3: >10 cm or prevents daily activity; and Grade 4: Necrosis.
Placebo
Dose 1
N=1114
-15.7
0.3
0
-0.5
0
0
-0.1
0
0
NVXCoV2373
Dose 2
N=2048
-61.4
2.1
0
-4.8
0.3
0
-5.4
0.4
0
Placebo
Dose 2
N=978
-16.5
0.1
0
-0.4
0
0
-0.4
0.1
0
21