よむ、つかう、まなぶ。
参考資料1-2: 令和3年度厚生労働科学特別研究事業「臨床研究法の見直しの審議における新たな課題・論点への対応策の確立のための研究」班 欧米での観察研究の取り扱い (8 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24643.html |
| 出典情報 | 厚生科学審議会 臨床研究部会(第29回 3/24)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Regulation (EU) No 536/2014 Questions & Answers – draft September 2021
Annex I: Decision tree to establish a whether a trial is a “clinical trial”
Note: this Annex and in particular the definition for a low-interventional trial are still under discussion in the expert group on clinical trials
This algorithm and its endnotes will help you answer that question. Please start in column A and follow the instructions. Additional information is provided in the notes at the end of the table. If you
have doubts about the answer to any of the questions contact the clinical trials unit of your competent authority.
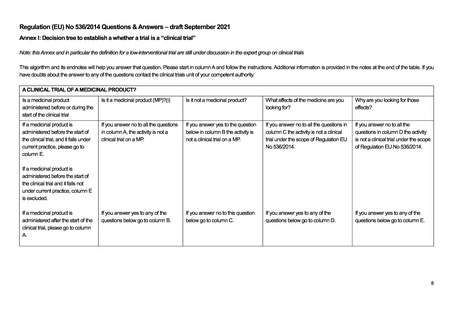
A CLINICAL TRIAL OF A MEDICINAL PRODUCT?
Is a medicinal product
administered before or during the
start of the clinical trial
Is it a medicinal product (MP)?(i)
Is it not a medicinal product?
What effects of the medicine are you
looking for?
Why are you looking for those
effects?
If a medicinal product is
administered before the start of
the clinical trial, and it falls under
current practice, please go to
column E.
If you answer no to all the questions
in column A, the activity is not a
clinical trial on a MP.
If you answer yes to the question
below in column B the activity is
not a clinical trial on a MP.
If you answer no to all the questions in
column C the activity is not a clinical
trial under the scope of Regulation EU
No 536/2014.
If you answer no to all the
questions in column D the activity
is not a clinical trial under the scope
of Regulation EU No 536/2014.
If you answer yes to any of the
questions below go to column B.
If you answer no to this question
below go to column C.
If you answer yes to any of the
questions below go to column D.
If you answer yes to any of the
questions below go to column E.
If a medicinal product is
administered before the start of
the clinical trial and it falls not
under current practice, column E
is excluded.
If a medicinal product is
administered after the start of the
clinical trial, please go to column
A.
8
Annex I: Decision tree to establish a whether a trial is a “clinical trial”
Note: this Annex and in particular the definition for a low-interventional trial are still under discussion in the expert group on clinical trials
This algorithm and its endnotes will help you answer that question. Please start in column A and follow the instructions. Additional information is provided in the notes at the end of the table. If you
have doubts about the answer to any of the questions contact the clinical trials unit of your competent authority.
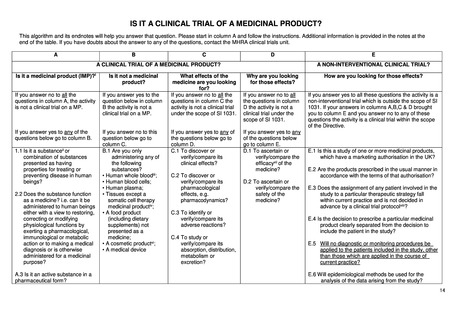
A CLINICAL TRIAL OF A MEDICINAL PRODUCT?
Is a medicinal product
administered before or during the
start of the clinical trial
Is it a medicinal product (MP)?(i)
Is it not a medicinal product?
What effects of the medicine are you
looking for?
Why are you looking for those
effects?
If a medicinal product is
administered before the start of
the clinical trial, and it falls under
current practice, please go to
column E.
If you answer no to all the questions
in column A, the activity is not a
clinical trial on a MP.
If you answer yes to the question
below in column B the activity is
not a clinical trial on a MP.
If you answer no to all the questions in
column C the activity is not a clinical
trial under the scope of Regulation EU
No 536/2014.
If you answer no to all the
questions in column D the activity
is not a clinical trial under the scope
of Regulation EU No 536/2014.
If you answer yes to any of the
questions below go to column B.
If you answer no to this question
below go to column C.
If you answer yes to any of the
questions below go to column D.
If you answer yes to any of the
questions below go to column E.
If a medicinal product is
administered before the start of
the clinical trial and it falls not
under current practice, column E
is excluded.
If a medicinal product is
administered after the start of the
clinical trial, please go to column
A.
8