よむ、つかう、まなぶ。
03【資料1】新型コロナワクチンの接種について (51 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29727.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第42回 12/13)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
参考資料一覧(2/5)
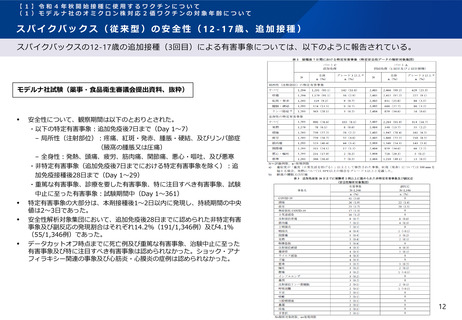
2022年秋以降の新型コロナワクチン追加接種及びオミクロン株対応ワクチンの接種に係る諸外国の状況 (2/2)
ドイツ
STIKO. 2022. Beschluss der STIKO zur 23. Aktualisierung der COVID-19-Impfempfehlung. [online] Available at: < https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2022/46/Art_01.html> [Accessed Dec 9, 2022]
イスラエル
Ministry of Health. 2022. HMOs Roll Out an Omicron-Specific Vaccine. [online] Available at: < https://www.gov.il/en/departments/news/20092022-04 > [Accessed 5 Oct 2022].
国際連合
WHO. 2022. Interim statement on the composition of current COVID-19 vaccines. [online] Available at: <https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Variants-2022.1> [Accessed 28
October 2022].
WHO. 2022. Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. [online] Available at: <https://www.who.int/news/item/17-05-2022-interim-statementon-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19> [Accessed 21 July 2022].
EU
EMA. 2022. ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines. [online] Available at: < https://www.ema.europa.eu/en/documents/public-statement/ecdc-ema-statementbooster-vaccination-omicron-adapted-bivalent-covid-19-vaccines_-0.pdf > [Accessed 13 Sep 2022].
EMA. 2022. Comirnaty. [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty > [Accessed 5 Oct 2022].
EMA. 2022. Spikevax (previously COVID-19 Vaccine Moderna). [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax> [Accessed 5 Oct 2022]
EMA. 2022. ETF concludes that bivalent original/Omicron BA.4-5 mRNA vaccines may be used for primary vaccination. [online] Available at: https://www.ema.europa.eu/en/news/etf-concludes-bivalent-originalomicron-ba4-5-mrna-vaccines-may-be-used-primary-vaccination [Accessed 9 Dec 2022]
51
2022年秋以降の新型コロナワクチン追加接種及びオミクロン株対応ワクチンの接種に係る諸外国の状況 (2/2)
ドイツ
STIKO. 2022. Beschluss der STIKO zur 23. Aktualisierung der COVID-19-Impfempfehlung. [online] Available at: < https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2022/46/Art_01.html> [Accessed Dec 9, 2022]
イスラエル
Ministry of Health. 2022. HMOs Roll Out an Omicron-Specific Vaccine. [online] Available at: < https://www.gov.il/en/departments/news/20092022-04 > [Accessed 5 Oct 2022].
国際連合
WHO. 2022. Interim statement on the composition of current COVID-19 vaccines. [online] Available at: <https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Variants-2022.1> [Accessed 28
October 2022].
WHO. 2022. Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. [online] Available at: <https://www.who.int/news/item/17-05-2022-interim-statementon-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19> [Accessed 21 July 2022].
EU
EMA. 2022. ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines. [online] Available at: < https://www.ema.europa.eu/en/documents/public-statement/ecdc-ema-statementbooster-vaccination-omicron-adapted-bivalent-covid-19-vaccines_-0.pdf > [Accessed 13 Sep 2022].
EMA. 2022. Comirnaty. [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty > [Accessed 5 Oct 2022].
EMA. 2022. Spikevax (previously COVID-19 Vaccine Moderna). [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax> [Accessed 5 Oct 2022]
EMA. 2022. ETF concludes that bivalent original/Omicron BA.4-5 mRNA vaccines may be used for primary vaccination. [online] Available at: https://www.ema.europa.eu/en/news/etf-concludes-bivalent-originalomicron-ba4-5-mrna-vaccines-may-be-used-primary-vaccination [Accessed 9 Dec 2022]
51