よむ、つかう、まなぶ。
【参考資料5】海外で臨床開発が先行した医薬品の国際共同治験開始前の日本人での第Ⅰ相試験の実施に関する基本的考え方について (9 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_41120.html |
| 出典情報 | 医薬品等行政評価・監視委員会(第16回 7/4)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
phase 1 study is conducted as an MRCT to collect information on Japanese, such as
pharmacokinetics (PK), as much as possible to provide detailed information to study
sites that will participate in the MRCTs and to appropriately design subsequent MRCTs
taking into account potential regional differences in intrinsic ethnic factors such as PK
that may affect the efficacy and safety of the drug.
For this reason, it is necessary to make a judgment for each individual drug based on the
balance between items such as the magnitude of the risk of the drug, sensitivity to
ethnic factors, medical needs, and disadvantages of not participating in MRCTs from
Japan.
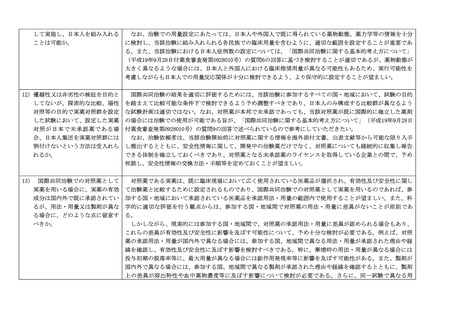
3. Examples of decisions for individual drugs
(1) Japan can participate in MRCTs without conducting a phase 1 study in Japanese
provided appropriate informed consent is obtained for drugs with high unmet
medical needs, such as drugs for rare diseases, diseases that are refractory and
serious, or pediatrics regardless of whether it is developed in adults, where
participation in planned or ongoing MRCTs is considered desirable to develop the
drug in Japan.
(2) Except for drugs described in (1), Japan can participate in MRCTs without
conducting a phase 1 study in Japanese if the safety of Japanese participants, at a
minimum, can be judged to be clinically acceptable/manageable considering facts
such as PK and/or response safety are less likely to be sensitive to ethnic factors
such as race based on non-clinical data, preceding foreign clinical trial results in
multiple races, available knowledge including information on similar drugs, and/or
modeling & simulation.
On the other hand, conduct of a phase 1 study in Japanese should be considered
when the study sponsor determines it is feasible in situations where the number of
patients in Japan is large and there is sufficient time to conduct a phase 1 study in
Japanese prior to the MRCTs. However, this does not apply when the risk in
Japanese is considered to be not significantly greater than in non-Japanese or when
the safety margin in humans is broad based on available information.
(3) Even for drugs that meet (1) or (2), the necessity of a phase 1 study in Japanese
should be judged more carefully if the drug is expected to frequently cause serious
adverse events and has a narrow safety margin, as observed for example in anticancer drugs, with limited safety data such as no experience of administration in
pharmacokinetics (PK), as much as possible to provide detailed information to study
sites that will participate in the MRCTs and to appropriately design subsequent MRCTs
taking into account potential regional differences in intrinsic ethnic factors such as PK
that may affect the efficacy and safety of the drug.
For this reason, it is necessary to make a judgment for each individual drug based on the
balance between items such as the magnitude of the risk of the drug, sensitivity to
ethnic factors, medical needs, and disadvantages of not participating in MRCTs from
Japan.
3. Examples of decisions for individual drugs
(1) Japan can participate in MRCTs without conducting a phase 1 study in Japanese
provided appropriate informed consent is obtained for drugs with high unmet
medical needs, such as drugs for rare diseases, diseases that are refractory and
serious, or pediatrics regardless of whether it is developed in adults, where
participation in planned or ongoing MRCTs is considered desirable to develop the
drug in Japan.
(2) Except for drugs described in (1), Japan can participate in MRCTs without
conducting a phase 1 study in Japanese if the safety of Japanese participants, at a
minimum, can be judged to be clinically acceptable/manageable considering facts
such as PK and/or response safety are less likely to be sensitive to ethnic factors
such as race based on non-clinical data, preceding foreign clinical trial results in
multiple races, available knowledge including information on similar drugs, and/or
modeling & simulation.
On the other hand, conduct of a phase 1 study in Japanese should be considered
when the study sponsor determines it is feasible in situations where the number of
patients in Japan is large and there is sufficient time to conduct a phase 1 study in
Japanese prior to the MRCTs. However, this does not apply when the risk in
Japanese is considered to be not significantly greater than in non-Japanese or when
the safety margin in humans is broad based on available information.
(3) Even for drugs that meet (1) or (2), the necessity of a phase 1 study in Japanese
should be judged more carefully if the drug is expected to frequently cause serious
adverse events and has a narrow safety margin, as observed for example in anticancer drugs, with limited safety data such as no experience of administration in