よむ、つかう、まなぶ。
資料3-3 ストラテラカプセル及びストラテラ内用液にて検出された新規ニトロソアミンの限度値について(企業見解)[7.8MB] (14 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_42464.html |
| 出典情報 | 薬事審議会 医薬品等安全対策部会安全対策調査会(令和6年度第5回 8/28)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Chemical Research in Toxicology
pubs.acs.org/crt
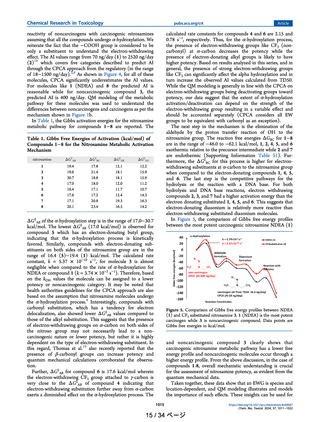
structures considered in the present study are classified into
three broad classes, alkyl, aryl, and alicyclic nitrosamines as
shown in Figure 3.
Article
This classification is primarily based on the assumption that
the fundamental chemical subunits that are unique in terms of
reactivity can be derived from the complex nitrosamines, and
the effect of structural complexity on TD50 can be rationalized
and understood using the appropriate tools (a “bottom-up”
approach). For the subclass formation, we focus on the
reactivity of hydrogens on the carbon next to the nitroso group
(α-hydrogen atoms) within each subclass which has been
established to be the key step in the metabolic cascade of
nitrosamines.17 With this classification, we aim to show that
the effect of various substituents (i.e., electronic and steric
effects) on a particular subclass can be satisfactorily described
through careful modeling of reactivity using quantum
mechanics. For the brevity of the manuscript, we have focused
our discussion on some specific subclasses where diversity in
the molecules and dynamic range of the TD50s are available
(vide infra). A similar analysis could be expanded to the whole
nitrosamine data set. Furthermore, this approach is consistent
with the underpinnings of the CPCA framework in recent
guidance on nitrosamine AI limits but provides the structural
basis of the reactivity beyond the predefined substructurebased classes to determine individual AI substance-specific
limits.
■
RESULTS AND DISCUSSION
We utilized the quantum mechanical activation energies
corresponding to α-hydroxylation, aldehyde formation, diazonium intermediate formation, DNA activation, and hydrolysis
reactions to understand the carcinogenic potency trends. In
particular, the discussion is focused on the selected series of
compounds such as N-nitroso pyrrolidines, N-nitroso piperidine, N-nitroso piperazine, N-nitroso morpholine, N-nitroso
thiomorpholine, N-nitroso N-methyl aromatic, fluorine substituted nitrosamines, and substituted aliphatic nitrosamines
for which significant experimental data is available to
rationalize the trends.
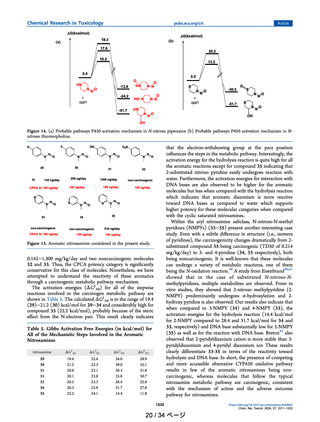
Substituent Effect in Dialkyl-Substituted Nitrosamines. Figure 4 represents nitrosamines with electrondonating substitution methyl (1), ethyl (4), propyl (5), and
electron-withdrawing CF3 (2, 3, 6), COCH3 (7), and COOH
(8) substitutions at α-carbon. Compounds 3 and 8 are
noncarcinogenic and are included in this study to compare the
Figure 3. Nitrosamines considered in the present work.
Figure 4. Nitrosamines illustrating electron withdrawing and electron donating substituent effects along with AI values based on TD50 values.19
The red color values show predicted AI values from the CPCA score.
1014
14 / 34 ページ
https://doi.org/10.1021/acs.chemrestox.4c00087
Chem. Res. Toxicol. 2024, 37, 1011−1022
pubs.acs.org/crt
structures considered in the present study are classified into
three broad classes, alkyl, aryl, and alicyclic nitrosamines as
shown in Figure 3.
Article
This classification is primarily based on the assumption that
the fundamental chemical subunits that are unique in terms of
reactivity can be derived from the complex nitrosamines, and
the effect of structural complexity on TD50 can be rationalized
and understood using the appropriate tools (a “bottom-up”
approach). For the subclass formation, we focus on the
reactivity of hydrogens on the carbon next to the nitroso group
(α-hydrogen atoms) within each subclass which has been
established to be the key step in the metabolic cascade of
nitrosamines.17 With this classification, we aim to show that
the effect of various substituents (i.e., electronic and steric
effects) on a particular subclass can be satisfactorily described
through careful modeling of reactivity using quantum
mechanics. For the brevity of the manuscript, we have focused
our discussion on some specific subclasses where diversity in
the molecules and dynamic range of the TD50s are available
(vide infra). A similar analysis could be expanded to the whole
nitrosamine data set. Furthermore, this approach is consistent
with the underpinnings of the CPCA framework in recent
guidance on nitrosamine AI limits but provides the structural
basis of the reactivity beyond the predefined substructurebased classes to determine individual AI substance-specific
limits.
■
RESULTS AND DISCUSSION
We utilized the quantum mechanical activation energies
corresponding to α-hydroxylation, aldehyde formation, diazonium intermediate formation, DNA activation, and hydrolysis
reactions to understand the carcinogenic potency trends. In
particular, the discussion is focused on the selected series of
compounds such as N-nitroso pyrrolidines, N-nitroso piperidine, N-nitroso piperazine, N-nitroso morpholine, N-nitroso
thiomorpholine, N-nitroso N-methyl aromatic, fluorine substituted nitrosamines, and substituted aliphatic nitrosamines
for which significant experimental data is available to
rationalize the trends.
Substituent Effect in Dialkyl-Substituted Nitrosamines. Figure 4 represents nitrosamines with electrondonating substitution methyl (1), ethyl (4), propyl (5), and
electron-withdrawing CF3 (2, 3, 6), COCH3 (7), and COOH
(8) substitutions at α-carbon. Compounds 3 and 8 are
noncarcinogenic and are included in this study to compare the
Figure 3. Nitrosamines considered in the present work.
Figure 4. Nitrosamines illustrating electron withdrawing and electron donating substituent effects along with AI values based on TD50 values.19
The red color values show predicted AI values from the CPCA score.
1014
14 / 34 ページ
https://doi.org/10.1021/acs.chemrestox.4c00087
Chem. Res. Toxicol. 2024, 37, 1011−1022