よむ、つかう、まなぶ。
資料3-3 ストラテラカプセル及びストラテラ内用液にて検出された新規ニトロソアミンの限度値について(企業見解)[7.8MB] (28 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_42464.html |
| 出典情報 | 薬事審議会 医薬品等安全対策部会安全対策調査会(令和6年度第5回 8/28)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
R.A. Jolly et al.
Regulatory Toxicology and Pharmacology 152 (2024) 105672
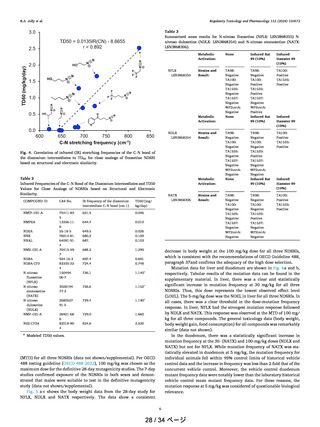
Table 3
Summarized ames results for N-nitroso fluoxetine (NFLX: LSN3868255) Nnitroso duloxetine (NDLX: LSN3868254) and N-nitroso atomoxetine (NATX:
LSN3868306).
NFLX
LSN3868255
Metabolic
Activation:
None
Induced Rat
S9 (10%)
Induced
Hamster S9
(10%)
Strains and
Result:
TA98:
Negative
TA100:
Negative
TA1535:
Negative
TA1537:
Negative
WP2uvrA:
Negative
None
TA98:
Negative
TA100:
Positive
TA1535:
Positive
TA1537:
Negative
WP2uvrA:
Positive
Induced Rat
S9 (10%)
TA100:
Positive
TA1535:
Positive
TA98:
Negative
TA100:
Negative
TA1535:
Negative
TA1537:
Negative
WP2uvrA:
Negative
None
TA98:
Negative
TA100:
Negative
TA1535:
Positive
TA1537:
Negative
WP2uvrA:
Negative
Induced Rat
S9 (10%)
TA100:
Positive
TA1535:
Positive
TA98:
Negative
TA100:
Negative
TA1535:
Negative
TA1537:
Negative
WP2uvrA:
Negative
TA98:
Negative
TA100:
Negative
TA1535:
Positive
TA1537:
Negative
WP2uvrA:
Negative
TA100:
Positive
TA1535:
Positive
Metabolic
Activation:
NDLX
LSN3868254
Strains and
Result:
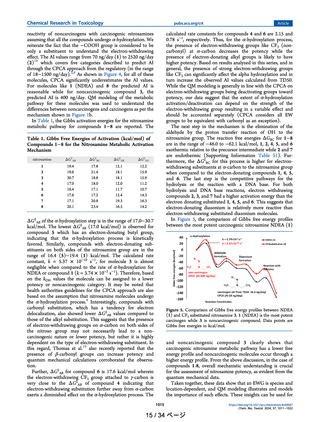
Fig. 4. Correlation of infrared (IR) stretching frequencies of the C–N bond of
the diazonium intermediates to TD50 for close analogs of fluoxetine NDSRI
based on structural and electronic similarity.
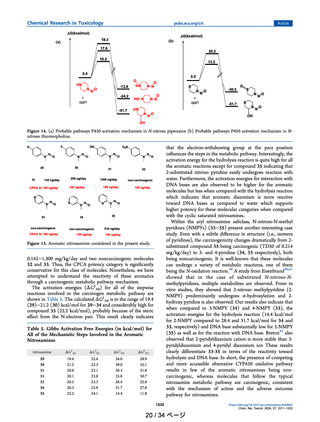
Table 2
Infrared Frequencies of the C–N Bond of the Diazonium Intermediates and TD50
Values for Close Analogs of NDSRIs based on Structural and Electronic
Similarity.
COMPOUND ID
CAS No.
IR frequency of the diazonium
intermediate C–N bond (cm-1)
TD50 (mg/
kg/day)
NMIP–OH–A
75411-835
13256-116
55-18-5
76014-8164091-914
70415-597
924-16-3
83335-324
15049406-7
621.5
0.046
644.4
0.010
649.5
680.2
687.
0.026
0.100
0.103
688.3
1.290
697.4
724.4
0.691
0.748
726.1
1.140a
302819477-3
726.6
1.152a
268052791-5
729.4
1.190a
26921-686
82018-904
729.0
1.660
824.6
2.520
NMPEA
NDEA
NNK
NNAL
NME–OH–A
NDBA
NDBA-CF3
N-nitroso
fluoxetine
(NFLX)
N-nitroso
atomoxetine
(NATX)
N-nitroso
duloxetine
(NDLX)
NMP–OH–A
NEE-CF3A
a
Metabolic
Activation:
NATX
LSN3868306
Strains and
Result:
Induced
Hamster S9
(10%)
Induced
Hamster S9
(10%)
decrease in body weight at the 100 mg/kg dose for all three NDSRIs,
which is consistent with the recommendations of OECD Guideline 488,
paragraph 37and confirms the adequacy of the high dose selection.
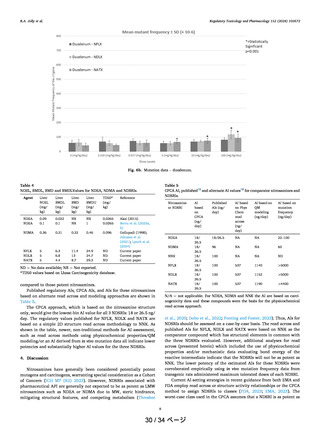
Mutation data for liver and duodenum are shown in Fig. 6a and b,
respectively. Tabular results of the mutation data can be found in the
supplementary material. In liver, there was a clear and statistically
significant increase in mutation frequency at 30 mg/kg for all three
NDSRIs. Thus, this dose represents the lowest observed effect level
(LOEL). The 5-mg/kg dose was the NOEL in liver for all three NDSRIs. In
all cases, there was a clear threshold in the dose-mutation frequency
response. In liver, NFLX had the strongest mutation response followed
by NDLX and NATX. This response was observed at the MTD of 100 mg/
kg for all three compounds. The general toxicology data (body weight,
body weight gain, food consumption) for all compounds was remarkably
similar (data not shown).
In the duodenum, there was a statistically significant increase in
mutation frequency at the 30- (NATX) and 100-mg/kg doses (NDLX and
NATX) but not for NFLX. While mutation frequency of NATX was statistically elevated in duodenum at 5 mg/kg, the mutation frequency for
individual animals fell within 95% control limits of historical vehicle
control data and the increase in frequency was less than 2-fold that of the
concurrent vehicle control. Moreover, the vehicle control duodenum
mutant frequency data were notably lower than the laboratory historical
vehicle control mean mutant frequency data. For these reasons, the
mutation response at 5 mg/kg was considered of questionable biological
relevance.
Modeled TD50 values.
(MTD) for all three NDSRIs (data not shown/supplemental). Per OECD
488 testing guideline (OECD 488 2022), 100 mg/kg was chosen as the
maximum dose for the definitive 28-day mutagenicity studies. The 7-day
studies confirmed exposure of the NDSRIs in both sexes and demonstrated that males were suitable to test in the definitive mutagenicity
study (data not shown/supplemental).
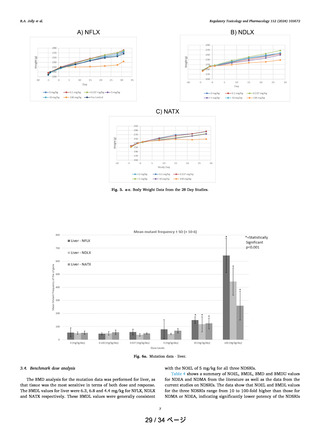
Fig. 5 a-c shows the body weight data from the 28-day study for
NFLX, NDLX and NATX respectively. The data show a consistent
6
28 / 34 ページ
Regulatory Toxicology and Pharmacology 152 (2024) 105672
Table 3
Summarized ames results for N-nitroso fluoxetine (NFLX: LSN3868255) Nnitroso duloxetine (NDLX: LSN3868254) and N-nitroso atomoxetine (NATX:
LSN3868306).
NFLX
LSN3868255
Metabolic
Activation:
None
Induced Rat
S9 (10%)
Induced
Hamster S9
(10%)
Strains and
Result:
TA98:
Negative
TA100:
Negative
TA1535:
Negative
TA1537:
Negative
WP2uvrA:
Negative
None
TA98:
Negative
TA100:
Positive
TA1535:
Positive
TA1537:
Negative
WP2uvrA:
Positive
Induced Rat
S9 (10%)
TA100:
Positive
TA1535:
Positive
TA98:
Negative
TA100:
Negative
TA1535:
Negative
TA1537:
Negative
WP2uvrA:
Negative
None
TA98:
Negative
TA100:
Negative
TA1535:
Positive
TA1537:
Negative
WP2uvrA:
Negative
Induced Rat
S9 (10%)
TA100:
Positive
TA1535:
Positive
TA98:
Negative
TA100:
Negative
TA1535:
Negative
TA1537:
Negative
WP2uvrA:
Negative
TA98:
Negative
TA100:
Negative
TA1535:
Positive
TA1537:
Negative
WP2uvrA:
Negative
TA100:
Positive
TA1535:
Positive
Metabolic
Activation:
NDLX
LSN3868254
Strains and
Result:
Fig. 4. Correlation of infrared (IR) stretching frequencies of the C–N bond of
the diazonium intermediates to TD50 for close analogs of fluoxetine NDSRI
based on structural and electronic similarity.
Table 2
Infrared Frequencies of the C–N Bond of the Diazonium Intermediates and TD50
Values for Close Analogs of NDSRIs based on Structural and Electronic
Similarity.
COMPOUND ID
CAS No.
IR frequency of the diazonium
intermediate C–N bond (cm-1)
TD50 (mg/
kg/day)
NMIP–OH–A
75411-835
13256-116
55-18-5
76014-8164091-914
70415-597
924-16-3
83335-324
15049406-7
621.5
0.046
644.4
0.010
649.5
680.2
687.
0.026
0.100
0.103
688.3
1.290
697.4
724.4
0.691
0.748
726.1
1.140a
302819477-3
726.6
1.152a
268052791-5
729.4
1.190a
26921-686
82018-904
729.0
1.660
824.6
2.520
NMPEA
NDEA
NNK
NNAL
NME–OH–A
NDBA
NDBA-CF3
N-nitroso
fluoxetine
(NFLX)
N-nitroso
atomoxetine
(NATX)
N-nitroso
duloxetine
(NDLX)
NMP–OH–A
NEE-CF3A
a
Metabolic
Activation:
NATX
LSN3868306
Strains and
Result:
Induced
Hamster S9
(10%)
Induced
Hamster S9
(10%)
decrease in body weight at the 100 mg/kg dose for all three NDSRIs,
which is consistent with the recommendations of OECD Guideline 488,
paragraph 37and confirms the adequacy of the high dose selection.
Mutation data for liver and duodenum are shown in Fig. 6a and b,
respectively. Tabular results of the mutation data can be found in the
supplementary material. In liver, there was a clear and statistically
significant increase in mutation frequency at 30 mg/kg for all three
NDSRIs. Thus, this dose represents the lowest observed effect level
(LOEL). The 5-mg/kg dose was the NOEL in liver for all three NDSRIs. In
all cases, there was a clear threshold in the dose-mutation frequency
response. In liver, NFLX had the strongest mutation response followed
by NDLX and NATX. This response was observed at the MTD of 100 mg/
kg for all three compounds. The general toxicology data (body weight,
body weight gain, food consumption) for all compounds was remarkably
similar (data not shown).
In the duodenum, there was a statistically significant increase in
mutation frequency at the 30- (NATX) and 100-mg/kg doses (NDLX and
NATX) but not for NFLX. While mutation frequency of NATX was statistically elevated in duodenum at 5 mg/kg, the mutation frequency for
individual animals fell within 95% control limits of historical vehicle
control data and the increase in frequency was less than 2-fold that of the
concurrent vehicle control. Moreover, the vehicle control duodenum
mutant frequency data were notably lower than the laboratory historical
vehicle control mean mutant frequency data. For these reasons, the
mutation response at 5 mg/kg was considered of questionable biological
relevance.
Modeled TD50 values.
(MTD) for all three NDSRIs (data not shown/supplemental). Per OECD
488 testing guideline (OECD 488 2022), 100 mg/kg was chosen as the
maximum dose for the definitive 28-day mutagenicity studies. The 7-day
studies confirmed exposure of the NDSRIs in both sexes and demonstrated that males were suitable to test in the definitive mutagenicity
study (data not shown/supplemental).
Fig. 5 a-c shows the body weight data from the 28-day study for
NFLX, NDLX and NATX respectively. The data show a consistent
6
28 / 34 ページ