よむ、つかう、まなぶ。
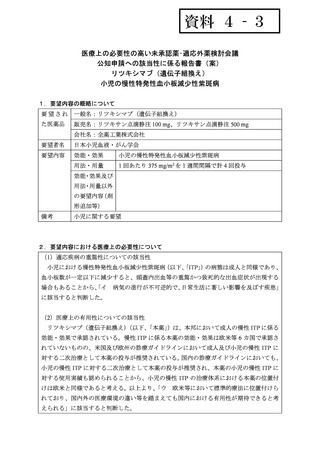
資料4-3 リツキシマブ(遺伝子組換え) (20 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00032.html |
| 出典情報 | 医薬・生活衛生局が実施する検討会 医療上の必要性の高い未承認薬・適応外薬検討会議(第58回 3/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
要望番号;IV-87
primary immune thrombocytopenia. Blood Adv 2019; 3: 3780-817.
7)
Neunert C, et al. American Society of Hematology 2019 guidelines for immune
thrombocytopenia. Blood Adv. 2019; 3: 3829-66.
8)
Grace RF, et al. Response to steroids predicts response to rituximab in pediatric chronic immune
thrombocytopenia. Pediatr Blood Cancer. 2012; 58: 221-5.
9)
Parodi E, et al. Long-term follow-up analysis after rituximab therapy in children with refractory
symptomatic ITP: identification of factors predictive of a sustained response. Br J Haematol
2009; 144: 552-8.
10) Mueller BU, et al. One year follow-up of children and adolescents with chronic immune
thrombocytopenic purpura (ITP) treated with rituximab. Pediatr Blood Cancer. 2009; 52: 25962.
11) Dierickx D, et al. Rituximab in auto-immune haemolytic anaemia and immune
thrombocytopenic purpura: a Belgian retrospective multicentric study. J Intern Med 2009; 266:
484-91.
12) Patel VL, et al. Outcomes 5 years after response to rituximab therapy in children and adults with
immune thrombocytopenia. Blood. 2012; 119:5989-95.
13) Oved JH, et al. Treatment of Children with Persistent and Chronic Idiopathic Thrombocytopenic
Purpura: 4 Infusions of Rituximab and Three 4-Day Cycles of Dexamethasone. J Pediatr 2017;
191: 225-31.
14) Liang Y, et al. Rituximab for children with immune thrombocytopenia: a systematic review.
PLoS One. 2012; 7: e36698.
15) Dai WJ, et al. Efficacy of standard dose rituximab for refractory idiopathic thrombocytopenic
purpura in children. Eur Rev Med Pharmacol Sci. 2015; 19: 2379-83.
16) Bennett CM, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic
immune thrombocytopenic purpura. Blood. 2006; 107: 2639-42.
17) COMPENDIA TRANSPARENCY TRACKING FORM (INDICATION: Immune thromboc
ytopenia, previously treated [pediatric]): https://www.merative.com/micromedex-training-ce
nter/compendia.(最終確認日2024年2月14日)
18) Local Coverage Determination (LCD): Off-label Use of Rituximab and Rituximab Bios
imilars (L38920). Medicare Coverage Database. Available from: https://www.cms.gov/me
dicare-coverage-database/view/lcd.aspx?lcdid=38920&ver=11&keyword=rituximab&keyword
Type=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&contract
Option=all&sortBy=relevance&bc=1(最終確認日 2024 年 2 月 14 日)
19) Local Coverage Determination (LCD): Off-label Use of Rituximab and Rituximab Bios
imilars (A58582). Medicare Coverage Database. Available from: https://www.cms.gov/me
dicare-coverage-database/view/article.aspx?articleid=58582&ver=13&keyword=rituximab&ke
ywordType=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&c
20
91 / 213
primary immune thrombocytopenia. Blood Adv 2019; 3: 3780-817.
7)
Neunert C, et al. American Society of Hematology 2019 guidelines for immune
thrombocytopenia. Blood Adv. 2019; 3: 3829-66.
8)
Grace RF, et al. Response to steroids predicts response to rituximab in pediatric chronic immune
thrombocytopenia. Pediatr Blood Cancer. 2012; 58: 221-5.
9)
Parodi E, et al. Long-term follow-up analysis after rituximab therapy in children with refractory
symptomatic ITP: identification of factors predictive of a sustained response. Br J Haematol
2009; 144: 552-8.
10) Mueller BU, et al. One year follow-up of children and adolescents with chronic immune
thrombocytopenic purpura (ITP) treated with rituximab. Pediatr Blood Cancer. 2009; 52: 25962.
11) Dierickx D, et al. Rituximab in auto-immune haemolytic anaemia and immune
thrombocytopenic purpura: a Belgian retrospective multicentric study. J Intern Med 2009; 266:
484-91.
12) Patel VL, et al. Outcomes 5 years after response to rituximab therapy in children and adults with
immune thrombocytopenia. Blood. 2012; 119:5989-95.
13) Oved JH, et al. Treatment of Children with Persistent and Chronic Idiopathic Thrombocytopenic
Purpura: 4 Infusions of Rituximab and Three 4-Day Cycles of Dexamethasone. J Pediatr 2017;
191: 225-31.
14) Liang Y, et al. Rituximab for children with immune thrombocytopenia: a systematic review.
PLoS One. 2012; 7: e36698.
15) Dai WJ, et al. Efficacy of standard dose rituximab for refractory idiopathic thrombocytopenic
purpura in children. Eur Rev Med Pharmacol Sci. 2015; 19: 2379-83.
16) Bennett CM, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic
immune thrombocytopenic purpura. Blood. 2006; 107: 2639-42.
17) COMPENDIA TRANSPARENCY TRACKING FORM (INDICATION: Immune thromboc
ytopenia, previously treated [pediatric]): https://www.merative.com/micromedex-training-ce
nter/compendia.(最終確認日2024年2月14日)
18) Local Coverage Determination (LCD): Off-label Use of Rituximab and Rituximab Bios
imilars (L38920). Medicare Coverage Database. Available from: https://www.cms.gov/me
dicare-coverage-database/view/lcd.aspx?lcdid=38920&ver=11&keyword=rituximab&keyword
Type=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&contract
Option=all&sortBy=relevance&bc=1(最終確認日 2024 年 2 月 14 日)
19) Local Coverage Determination (LCD): Off-label Use of Rituximab and Rituximab Bios
imilars (A58582). Medicare Coverage Database. Available from: https://www.cms.gov/me
dicare-coverage-database/view/article.aspx?articleid=58582&ver=13&keyword=rituximab&ke
ywordType=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&c
20
91 / 213