よむ、つかう、まなぶ。
04【資料2】新型コロナワクチンの接種について (80 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000192554_00021.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第31回 3/24)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
参考資料一覧(4/6)
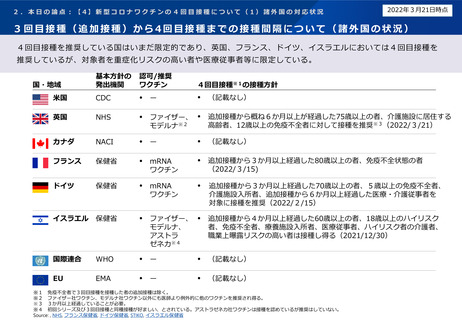
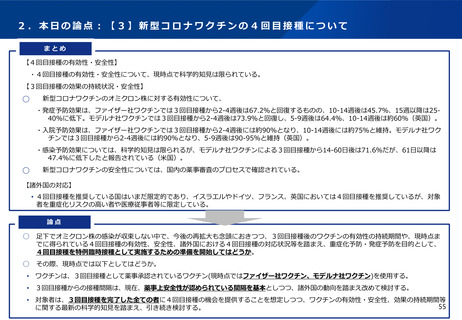
諸外国における新型コロナワクチン追加接種の状況について
米国
•
CDC. Guidance for COVID-19 vaccination for people who are moderately or severely immunocompromised. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised [Accessed
Mar 18, 2022]
•
保健福祉省 Statement by HHS Secretary Xavier Becerra on COVID-19 Vaccine Booster Doses Published Sep 24, 2021. https://www.hhs.gov/about/news/2021/09/24/statement-by-hhs-secretary-xavier-becerra-covid-19-vaccine-boosterdoses.html [Accessed Mar 18, 2022]
•
CDC. COVID-19 Vaccine Booster Shots 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html [Accessed Mar 18, 2022].
•
FDA. Evaluation of a Booster Dose (Third Dose) Vaccines and Related Biological Products Advisory Committee Published Sep 17, 2021. https://www.fda.gov/media/152161/download [Accessed Mar 18, 2022].
•
CDC. CDC Expands Eligibility for COVID-19 Booster Shots. Published Oct 21, 2021. https://www.cdc.gov/media/releases/2021/p1021-covid-booster.html [Accessed Mar 18, 2022]
•
FDA. Coronavirus (COVID-19) Update: FDA Takes Additional Actions on the Use of a Booster Dose for COVID-19 Vaccines. Published Oct 20, 2021.
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines [Accessed Mar 18, 2022]
•
CDC. CDC Recommends Pfizer Booster at 5 Months, Additional Primary Dose for Certain Immunocompromised Children. [online] https://www.cdc.gov/media/releases/2022/s0104-PfizerBooster.html#:~:text=CDC%20Newsroom%20Releases,CDC%20Recommends%20Pfizer%20Booster%20at%205%20Months%2C%20Additional,Dose%20for%20Certain%20Immunocompromised%20Children&text=Today%2C%20CDC%20is%20updating%20our,-BioNTech%20COVID19%20Vaccine. [Accessed Mar 18, 2022].
•
CDC. CDC Expands COVID-19 Booster Recommendations to 16-and-17-year-olds. Published Dec 9, 2021. https://www.cdc.gov/media/releases/2021/s1208-16-17-booster.html. [Accessed Mar 18, 2022]
•
CDC. CDC Expands Booster Shot Eligibility and Strengthens Recommendations for 12-17 Year Olds. Published Jan 5, 2022. https://www.cdc.gov/media/releases/2022/s0105-Booster-Shot.html. [Accessed Mar 18, 2022]
英国
•
英国内閣府 COVID-19 RESPOSE: AUTUM AND WINTER PLAN Published Sep 14, 2021 https://www.gov.uk/government/publications/covid-19-response-autumn-and-winter-plan-2021/covid-19-response-autumn-and-winter-plan-2021
[Accessed Mar 18, 2022]
•
JCVI updated advice on COVID-19 booster vaccination Sep 14, 2021 https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-booster-vaccination [Accessed Sep 15 2021]
•
NHS. How to get a booster dose of the coronavirus (COVID-19) vaccine. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/how-to-get-a-coronavirus-vaccine/how-to-get-a-booster-dose/ [Accessed Mar 18,
2022].
•
GOV.UK. 2021. JCVI advice on COVID-19 booster vaccines for those aged 18 to 39 and a second dose for ages 12 to 15. https://www.gov.uk/government/news/jcvi-advice-on-covid-19-booster-vaccines-for-those-aged-18-to-39-and-asecond-dose-for-ages-12-to-15 [Accessed Mar 18, 2022].
•
GOV.UK. 2021. All adults to be offered COVID-19 boosters by end of January. https://www.gov.uk/government/news/all-adults-to-be-offered-covid-19-boosters-by-end-of-january [Accessed Mar 18, 2022].
•
GOV.UK. 2021. Prime Minister’s address to the nation on booster jabs: 12 December 2021. https://www.gov.uk/government/speeches/prime-ministers-address-to-the-nation-on-booster-jabs-12-december-2021 [Accessed Mar 18, 2022].
•
NHS England. 2022. Hundreds of thousands of teens to get boosted on NHS. https://www.england.nhs.uk/2022/01/hundreds-of-thousands-of-teens-to-get-boosted-on-nhs-2/ [Accessed Mar 18, 2022].
•
GOV.UK. 2022. JCVI statement on the adult COVID-19 booster vaccination programme and the Omicron variant: 7 January 2022. https://www.gov.uk/government/publications/jcvi-statement-on-the-adult-covid-19-booster-vaccinationprogramme-and-the-omicron-variant/jcvi-statement-on-the-adult-covid-19-booster-vaccination-programme-and-the-omicron-variant-7-january-2022 [Accessed Mar 18, 2022].
•
NHS. 2022. A guide to the spring booster for those aged 75 years and older residents in care homes. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1061917/UKHSA-12308-COVID19-spring-booster-guide-for-over-75s-v2.pdf [Accessed Mar 18, 2022]
カナダ
•
カナダ政府 (NACI). Summary of NACI rapid response. Updated Sep 10, 2021 https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/summary-september-10-2021-additionaldose-covid-19-vaccine-immunocompromised-following-1-2-dose-series.html [Accessed Sep 28, 2021]
•
カナダ政府 (NACI). NACI rapid response: Booster dose in long-term care residents and seniors living in other congregate settings. Published Sep 28, 2021 https://www.canada.ca/en/public-health/services/immunization/national-advisorycommittee-on-immunization-naci/statement-september-28-2021-booster-dose-long-term-care-residents-seniors-living-other-congregate-settings.html [Accessed Oct 5, 2021]
•
カナダ政府 (NACI). Recommendations on the use of COVID-19 vaccines. Updated Oct 22, 2021 https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunizationnaci/recommendations-use-covid-19-vaccines/recommendations-use-covid-19-vaccines-en.pdf [Accessed Oct 23, 2021]
•
PHAC. SUMMARY OF NATIONAL ADVISORY COMMITTEE ON IMMUNIZATION (NACI) STATEMENT OF OCTOBER 29, 2021. Updated Oct 29, 2021 https://www.canada.ca/content/dam/phacaspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/statement-guidance-booster-doses/summary/summary.pdf [Accessed Nov 4, 2021]
•
PHAC. 2021. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Guidance on booster COVID-19 vaccine doses in Canada – Update December 3, 2021.
https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-booster-covid-19-vaccine-doses/guidance-booster-covid-19-vaccine-doses.pdf [Accessed
December 13, 2021].
•
CTV News. 2022. How long should you wait for your third COVID-19 vaccine dose?. [online] Available at: <https://www.ctvnews.ca/health/coronavirus/how-long-should-you-wait-for-your-third-covid-19-vaccine-dose-1.5722476>
[Accessed January 17, 2022].
•
カナダ政府 (NACI). Vaccines for COVID-19: How to get vaccinated - Booster doses. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/vaccines/how-vaccinated.html#a9 [Accessed Mar 18, 2022]
•
カナダ政府 (NACI). Rapid response: Guidance on the use of booster COVID-19 vaccine doses in adolescents 12-17 years of age[online] Available at: <https://www.canada.ca/content/dam/phacaspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-use-booster-covid-19-vaccines-adolescents-12-17-years-age.pdf> [Accessed 7 February 2022].
•
カナダ政府 (NACI). Recommendations on the use of Novavax Nuvaxovid COVID-19 vaccine. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-usenovavax-nuvaxovid-covid-19-vaccine.html#a7 [Accessed Mar 18, 2022]
80
諸外国における新型コロナワクチン追加接種の状況について
米国
•
CDC. Guidance for COVID-19 vaccination for people who are moderately or severely immunocompromised. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised [Accessed
Mar 18, 2022]
•
保健福祉省 Statement by HHS Secretary Xavier Becerra on COVID-19 Vaccine Booster Doses Published Sep 24, 2021. https://www.hhs.gov/about/news/2021/09/24/statement-by-hhs-secretary-xavier-becerra-covid-19-vaccine-boosterdoses.html [Accessed Mar 18, 2022]
•
CDC. COVID-19 Vaccine Booster Shots 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html [Accessed Mar 18, 2022].
•
FDA. Evaluation of a Booster Dose (Third Dose) Vaccines and Related Biological Products Advisory Committee Published Sep 17, 2021. https://www.fda.gov/media/152161/download [Accessed Mar 18, 2022].
•
CDC. CDC Expands Eligibility for COVID-19 Booster Shots. Published Oct 21, 2021. https://www.cdc.gov/media/releases/2021/p1021-covid-booster.html [Accessed Mar 18, 2022]
•
FDA. Coronavirus (COVID-19) Update: FDA Takes Additional Actions on the Use of a Booster Dose for COVID-19 Vaccines. Published Oct 20, 2021.
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines [Accessed Mar 18, 2022]
•
CDC. CDC Recommends Pfizer Booster at 5 Months, Additional Primary Dose for Certain Immunocompromised Children. [online] https://www.cdc.gov/media/releases/2022/s0104-PfizerBooster.html#:~:text=CDC%20Newsroom%20Releases,CDC%20Recommends%20Pfizer%20Booster%20at%205%20Months%2C%20Additional,Dose%20for%20Certain%20Immunocompromised%20Children&text=Today%2C%20CDC%20is%20updating%20our,-BioNTech%20COVID19%20Vaccine. [Accessed Mar 18, 2022].
•
CDC. CDC Expands COVID-19 Booster Recommendations to 16-and-17-year-olds. Published Dec 9, 2021. https://www.cdc.gov/media/releases/2021/s1208-16-17-booster.html. [Accessed Mar 18, 2022]
•
CDC. CDC Expands Booster Shot Eligibility and Strengthens Recommendations for 12-17 Year Olds. Published Jan 5, 2022. https://www.cdc.gov/media/releases/2022/s0105-Booster-Shot.html. [Accessed Mar 18, 2022]
英国
•
英国内閣府 COVID-19 RESPOSE: AUTUM AND WINTER PLAN Published Sep 14, 2021 https://www.gov.uk/government/publications/covid-19-response-autumn-and-winter-plan-2021/covid-19-response-autumn-and-winter-plan-2021
[Accessed Mar 18, 2022]
•
JCVI updated advice on COVID-19 booster vaccination Sep 14, 2021 https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-booster-vaccination [Accessed Sep 15 2021]
•
NHS. How to get a booster dose of the coronavirus (COVID-19) vaccine. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/how-to-get-a-coronavirus-vaccine/how-to-get-a-booster-dose/ [Accessed Mar 18,
2022].
•
GOV.UK. 2021. JCVI advice on COVID-19 booster vaccines for those aged 18 to 39 and a second dose for ages 12 to 15. https://www.gov.uk/government/news/jcvi-advice-on-covid-19-booster-vaccines-for-those-aged-18-to-39-and-asecond-dose-for-ages-12-to-15 [Accessed Mar 18, 2022].
•
GOV.UK. 2021. All adults to be offered COVID-19 boosters by end of January. https://www.gov.uk/government/news/all-adults-to-be-offered-covid-19-boosters-by-end-of-january [Accessed Mar 18, 2022].
•
GOV.UK. 2021. Prime Minister’s address to the nation on booster jabs: 12 December 2021. https://www.gov.uk/government/speeches/prime-ministers-address-to-the-nation-on-booster-jabs-12-december-2021 [Accessed Mar 18, 2022].
•
NHS England. 2022. Hundreds of thousands of teens to get boosted on NHS. https://www.england.nhs.uk/2022/01/hundreds-of-thousands-of-teens-to-get-boosted-on-nhs-2/ [Accessed Mar 18, 2022].
•
GOV.UK. 2022. JCVI statement on the adult COVID-19 booster vaccination programme and the Omicron variant: 7 January 2022. https://www.gov.uk/government/publications/jcvi-statement-on-the-adult-covid-19-booster-vaccinationprogramme-and-the-omicron-variant/jcvi-statement-on-the-adult-covid-19-booster-vaccination-programme-and-the-omicron-variant-7-january-2022 [Accessed Mar 18, 2022].
•
NHS. 2022. A guide to the spring booster for those aged 75 years and older residents in care homes. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1061917/UKHSA-12308-COVID19-spring-booster-guide-for-over-75s-v2.pdf [Accessed Mar 18, 2022]
カナダ
•
カナダ政府 (NACI). Summary of NACI rapid response. Updated Sep 10, 2021 https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/summary-september-10-2021-additionaldose-covid-19-vaccine-immunocompromised-following-1-2-dose-series.html [Accessed Sep 28, 2021]
•
カナダ政府 (NACI). NACI rapid response: Booster dose in long-term care residents and seniors living in other congregate settings. Published Sep 28, 2021 https://www.canada.ca/en/public-health/services/immunization/national-advisorycommittee-on-immunization-naci/statement-september-28-2021-booster-dose-long-term-care-residents-seniors-living-other-congregate-settings.html [Accessed Oct 5, 2021]
•
カナダ政府 (NACI). Recommendations on the use of COVID-19 vaccines. Updated Oct 22, 2021 https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunizationnaci/recommendations-use-covid-19-vaccines/recommendations-use-covid-19-vaccines-en.pdf [Accessed Oct 23, 2021]
•
PHAC. SUMMARY OF NATIONAL ADVISORY COMMITTEE ON IMMUNIZATION (NACI) STATEMENT OF OCTOBER 29, 2021. Updated Oct 29, 2021 https://www.canada.ca/content/dam/phacaspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/statement-guidance-booster-doses/summary/summary.pdf [Accessed Nov 4, 2021]
•
PHAC. 2021. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Guidance on booster COVID-19 vaccine doses in Canada – Update December 3, 2021.
https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-booster-covid-19-vaccine-doses/guidance-booster-covid-19-vaccine-doses.pdf [Accessed
December 13, 2021].
•
CTV News. 2022. How long should you wait for your third COVID-19 vaccine dose?. [online] Available at: <https://www.ctvnews.ca/health/coronavirus/how-long-should-you-wait-for-your-third-covid-19-vaccine-dose-1.5722476>
[Accessed January 17, 2022].
•
カナダ政府 (NACI). Vaccines for COVID-19: How to get vaccinated - Booster doses. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/vaccines/how-vaccinated.html#a9 [Accessed Mar 18, 2022]
•
カナダ政府 (NACI). Rapid response: Guidance on the use of booster COVID-19 vaccine doses in adolescents 12-17 years of age[online] Available at: <https://www.canada.ca/content/dam/phacaspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-use-booster-covid-19-vaccines-adolescents-12-17-years-age.pdf> [Accessed 7 February 2022].
•
カナダ政府 (NACI). Recommendations on the use of Novavax Nuvaxovid COVID-19 vaccine. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-usenovavax-nuvaxovid-covid-19-vaccine.html#a7 [Accessed Mar 18, 2022]
80