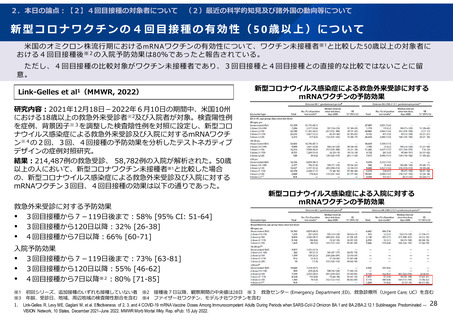
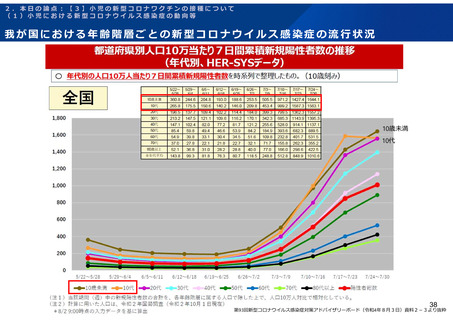
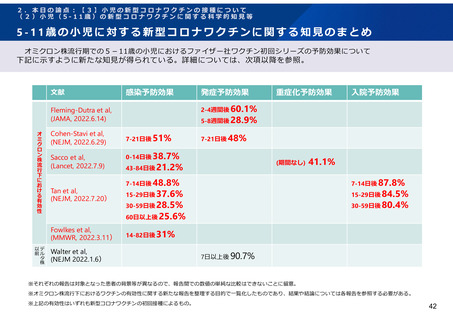
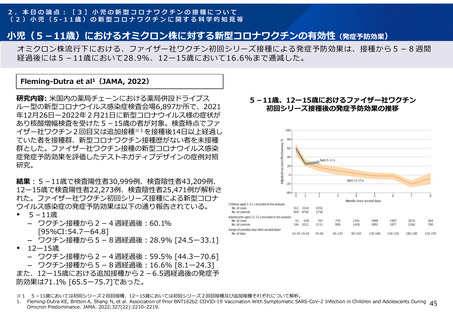
よむ、つかう、まなぶ。
03【資料1】新型コロナワクチンの接種について (74 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27303.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第34回 8/8)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
参考資料一覧(1/5)
オミクロン株対応ワクチンに係る諸外国の状況
2022年秋以降の新型コロナワクチン追加接種の諸外国の状況
米国
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses. [online] Available at: <https://www.fda.gov/news-events/pressannouncements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster> [Accessed 21 July 2022].
英国
GOV.UK. 2022. Joint Committee on Vaccination and Immunisation (JCVI) updated statement on the COVID-19 vaccination programme for autumn 2022. [online] Available at: <https://www.gov.uk/government/publications/jcvi-updated-statementon-the-covid-19-vaccination-programme-for-autumn-2022/joint-committee-on-vaccination-and-immunisation-jcvi-updated-statement-on-the-covid-19-vaccination-programme-for-autumn-2022> [Accessed 21 July 2022].
カナダ
NACI. 2022. Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada. [online] Available at: <https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisorycommittee-on-immunization-naci/naci-guidance-planning-fall-2022-covid-19-vaccine-booster.pdf> [Accessed 21 July 2022].
フランス
Haute Autorité de Santé. 2022. Avis n°2022.0036/AC/SESPEV du 16 juin 2022 du collège de la Haute Autorité de santé relatif au lancement de la campagne de vaccination 2022/2023 contre la grippe saisonnière en France dans l’hémisphère
Nord et à Mayotte dans le contexte de l’épidémie de Covid-19. [online] Available at: <https://www.has-sante.fr/jcms/p_3345196/fr/avis-n2022-0036/ac/sespev-du-16-juin-2022-du-college-de-la-haute-autorite-de-sante-relatif-au-lancement-de-lacampagne-de-vaccination-2022/2023-contre-la-grippe-saisonniere-en-france-dans-l-hemisphere-nord-et-a-mayotte-dans-le-contexte-de-l-epidemie-de-covid-19> [Accessed 21 July 2022].
イスラエル
Ministry of Health. 2022. [online] Available at: <https://www.gov.il/BlobFolder/reports/ect-12062022/he/files_publications_units_epidemic_control_ect-12062022.pdf> [Accessed 21 July 2022].
国際連合
WHO. 2022. Interim statement on the composition of current COVID-19 vaccines. [online] Available at: <https://www.who.int/news/item/17-06-2022-interim-statement-on--the-composition-of-current-COVID-19-vaccines> [Accessed 21 July 2022].
WHO. 2022. Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. [online] Available at: <https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additionalbooster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19> [Accessed 21 July 2022].
EU
EMA. 2022. ECDC and EMA update recommendations on additional booster doses of mRNA COVID-19 vaccines - European Medicines Agency. [online] Available at: <https://www.ema.europa.eu/en/news/ecdc-ema-update-recommendationsadditional-booster-doses-mrna-covid-19-vaccines> [Accessed 21 July 2022].
74
オミクロン株対応ワクチンに係る諸外国の状況
2022年秋以降の新型コロナワクチン追加接種の諸外国の状況
米国
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses. [online] Available at: <https://www.fda.gov/news-events/pressannouncements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster> [Accessed 21 July 2022].
英国
GOV.UK. 2022. Joint Committee on Vaccination and Immunisation (JCVI) updated statement on the COVID-19 vaccination programme for autumn 2022. [online] Available at: <https://www.gov.uk/government/publications/jcvi-updated-statementon-the-covid-19-vaccination-programme-for-autumn-2022/joint-committee-on-vaccination-and-immunisation-jcvi-updated-statement-on-the-covid-19-vaccination-programme-for-autumn-2022> [Accessed 21 July 2022].
カナダ
NACI. 2022. Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada. [online] Available at: <https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisorycommittee-on-immunization-naci/naci-guidance-planning-fall-2022-covid-19-vaccine-booster.pdf> [Accessed 21 July 2022].
フランス
Haute Autorité de Santé. 2022. Avis n°2022.0036/AC/SESPEV du 16 juin 2022 du collège de la Haute Autorité de santé relatif au lancement de la campagne de vaccination 2022/2023 contre la grippe saisonnière en France dans l’hémisphère
Nord et à Mayotte dans le contexte de l’épidémie de Covid-19. [online] Available at: <https://www.has-sante.fr/jcms/p_3345196/fr/avis-n2022-0036/ac/sespev-du-16-juin-2022-du-college-de-la-haute-autorite-de-sante-relatif-au-lancement-de-lacampagne-de-vaccination-2022/2023-contre-la-grippe-saisonniere-en-france-dans-l-hemisphere-nord-et-a-mayotte-dans-le-contexte-de-l-epidemie-de-covid-19> [Accessed 21 July 2022].
イスラエル
Ministry of Health. 2022. [online] Available at: <https://www.gov.il/BlobFolder/reports/ect-12062022/he/files_publications_units_epidemic_control_ect-12062022.pdf> [Accessed 21 July 2022].
国際連合
WHO. 2022. Interim statement on the composition of current COVID-19 vaccines. [online] Available at: <https://www.who.int/news/item/17-06-2022-interim-statement-on--the-composition-of-current-COVID-19-vaccines> [Accessed 21 July 2022].
WHO. 2022. Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. [online] Available at: <https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additionalbooster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19> [Accessed 21 July 2022].
EU
EMA. 2022. ECDC and EMA update recommendations on additional booster doses of mRNA COVID-19 vaccines - European Medicines Agency. [online] Available at: <https://www.ema.europa.eu/en/news/ecdc-ema-update-recommendationsadditional-booster-doses-mrna-covid-19-vaccines> [Accessed 21 July 2022].
74