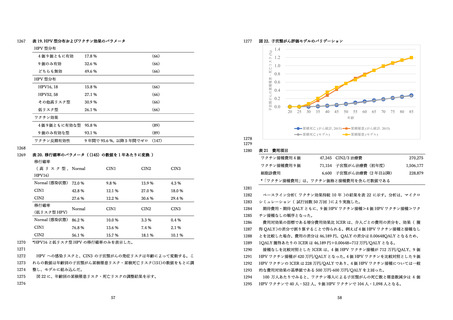
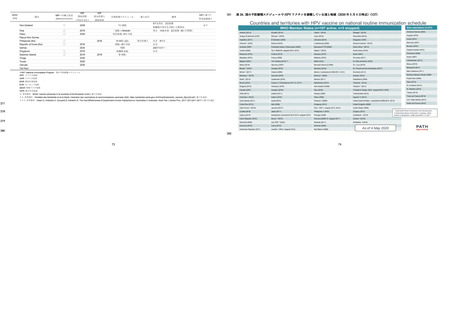
よむ、つかう、まなぶ。
11【参考資料1-6】9価HPVワクチンファクトシート (46 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29181.html |
| 出典情報 | 厚生科学審議会予防接種・ワクチン分科会(第41回 11/18)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
1912
神経 55:179-183 https://www.jstage.jst.go.jp/article/ans/56/3/56_93/_pdf/-char/ja
1948
1322.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4514333/pdf/khvi-4514311-451430
1913
127. Yokota, S., et al. (2018). Human papilloma virus(HPV) vaccination-associated neuro-
1949
6-1012010.pdf
1914
immunopathic syndrome(HANS): a unique symptomatic spectrum and the pathological role
1950
138.
1915
of hypothalamus. ⾃律神経 55(3): 171-178.
1951
effectiveness analysis of prophylactic cervical cancer vaccination in Japanese women.Int J
1916
http://search.jamas.or.jp/link/ui/2019282514
1952
Gynecol Cancer. 2010;20(3):385-92
1917
128. Aratani, S., et al. (2018). What occurs in human papilloma virus vaccination-associated
1953
139.
1918
neuro-immunopathic syndrome(HANS)?: implications of previous animal model studies. ⾃
1954
Introducing HPV vaccine and scaling up screening procedures to prevent deaths from cervical
1919
律神経 55(3): 165-117. http://search.jamas.or.jp/link/ui/2019282513.
1955
cancer in Japan: a cost-effectiveness analysis. Bjog. 2012;119(2):177-86.
1920
129. ⾼嶋 博.(2019).脳炎・脳症・脊髄症の新たな展開 ヒトパピローマウイルスワクチ
1956
140.
1921
ン接種後の神経症状は、なぜ⼼因性疾患と間違われるのか.神経治療学 2018;35:536-542.
1957
Effectiveness Analysis of a Quadrivalent Human Papillomavirus Vaccine (6/11/16/18) for
1922
http://search.jamas.or.jp/link/ui/2019260843
1958
Females in Japan.Value Health Reg Issues. 2013;2(1):92-7.
1923
130. Ushida, T., et al. (2016). The Effect of Guidance regarding Home Exercise and ADL
1959
141.
1924
on Adolescent Females Suffering from Adverse Effects after HPV Vaccination in Japanese
1960
effectiveness of routine and catch-up vaccination of girls and women with a nine-valent HPV
1925
Multidisciplinary Pain Centers. Pain Res Manag 2016: 3689352.
1961
vaccine in Japan: a model-based study. BMC Infect Dis. 2021;21(1):11
1926
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4904598/pdf/PRM3682016-3689352.pdf
1962
142 . Isshiki T. HPV vaccination for cervical cancer prevention is not cost-effective in Japan.
1927
131. WHO (2017). Human papillomavirus vaccines: WHO position paper, May 2017. Wkly
1963
Asian Pac J Cancer Prev. 2014; 15(15): 6177-80.
1928
Epidemiol Rec 92(19): 241-268.
1964
143 . Kitano T. Stopping the HPV vaccine crisis in Japan: Quantifying the benefits and risks
1929
132. Centers for Disease Control and Prevention.(2020). Vaccine for HPV.
1965
of HPV vaccination in quality-adjusted life-years for appropriate decision-making. J Infect
1930
https://www.cdc.gov/hpv/parents/vaccinesafety.html
1966
Chemother. 2020; 26(3): 225-30.
1931
133. Control, E. C. f. D. P. a. (2020). Guidance on HPV vaccination in EU countries: focus
1967
144. 厚⽣労働省. 第 14 回厚⽣科学審議会予防接種・ワクチン分科会予防接種基本⽅針部
1932
on boys, people living with HIV and 9-valent HPV vaccine introduction.
1968
会 資料 3 沈降13価肺炎球菌結合型ワクチンを⾼齢者へ定期接種で使⽤することの是⾮
1933
https://www.ecdc.europa.eu/sites/default/files/documents/Guidance-on-HPV-vaccination
1969
に関する検討⽅針について
1934
-in-EU-countries2020-03-30.pdf
1970
https://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-
1935
134. Agency, E. M. HPV vaccines: EMA confirms evidence does not support that they cause
1971
Kouseikagakuka/0000111764.pdf
1936
CRPS or POTS 2016.
1972
145. Taguchi, A., et al. (2020). Multistate Markov Model to Predict the Prognosis of High-
1937
http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vacc
1973
Risk Human Papillomavirus-Related Cervical Lesions. Cancers (Basel) 12(2).
1938
ines_20/European_Commission_final_decision/WC500196773.pdf
1974
146. 村澤秀樹 ⼤久保⼀郎, 荒川⼀郎. (2014). ⼦宮頸がん検診へのヒトパピローマウイル
1939
135. Zhang, Z., et al. (2017). Expanded strain coverage for a highly successful public health
1975
ス DNA 検査導⼊に関する費⽤対効果分析.⽇本予防医学会雑誌 2014;9 (2):83-91.
1940
tool: Prophylactic 9-valent human papillomavirus vaccine. Hum Vaccin Immunother 13(10):
1976
147. De Vincenzo, R., et al. (2014). Long-term efficacy and safety of human papillomavirus
1941
2280-2291. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5647960/pdf/khvi-5647913-
1977
vaccination. Int J Womens Health 6: 999-1010.
1942
5647910-1346755.pdf
1978
148. Datta, S., et al. (2019). Assessing the cost-effectiveness of HPV vaccination strategies
1943
136. Yang, D. Y. and K. Bracken (2016). Update on the new 9-valent vaccine for human
1979
for adolescent girls and boys in the UK. BMC Infect Dis 19(1): 552.
1944
papillomavirus prevention. Can Fam Physician 62(5): 399-402.
1980
149. Brisson, M., et al. (2016). Health and Economic Impact of Switching from a 4-Valent
1945
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865336/pdf/0620399.pdf
1981
to a 9-Valent HPV Vaccination Program in the United States. J Natl Cancer Inst 108(1).
1946
137.
Luxembourg, A., et al. (2015). Phase II studies to select the formulation of a
1982
150. Murasawa, H., et al. (2014). Evaluation of health-related quality of life for hypothesized
1947
multivalent HPV L1 virus-like particle (VLP) vaccine. Hum Vaccin Immunother 11(6): 1313-
1983
medical states associated with cervical cancer. Asian Pac J Cancer Prev 15(22): 9679-9685.
91
Konno R, Sasagawa T, Fukuda T, Van Kriekinge G, Demarteau N. (2010).Cost-
Yamamoto N, Mori R, Jacklin P, Osuga Y, Kawana K, Shibuya K, et al.(2012).
Yamabe K, Singhal PK, Abe M, Dasbach EJ, Elbasha EH. (2013).The Cost-
Cody P, Tobe K, Abe M, Elbasha EH.(2012). Public health impact and cost
92
神経 55:179-183 https://www.jstage.jst.go.jp/article/ans/56/3/56_93/_pdf/-char/ja
1948
1322.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4514333/pdf/khvi-4514311-451430
1913
127. Yokota, S., et al. (2018). Human papilloma virus(HPV) vaccination-associated neuro-
1949
6-1012010.pdf
1914
immunopathic syndrome(HANS): a unique symptomatic spectrum and the pathological role
1950
138.
1915
of hypothalamus. ⾃律神経 55(3): 171-178.
1951
effectiveness analysis of prophylactic cervical cancer vaccination in Japanese women.Int J
1916
http://search.jamas.or.jp/link/ui/2019282514
1952
Gynecol Cancer. 2010;20(3):385-92
1917
128. Aratani, S., et al. (2018). What occurs in human papilloma virus vaccination-associated
1953
139.
1918
neuro-immunopathic syndrome(HANS)?: implications of previous animal model studies. ⾃
1954
Introducing HPV vaccine and scaling up screening procedures to prevent deaths from cervical
1919
律神経 55(3): 165-117. http://search.jamas.or.jp/link/ui/2019282513.
1955
cancer in Japan: a cost-effectiveness analysis. Bjog. 2012;119(2):177-86.
1920
129. ⾼嶋 博.(2019).脳炎・脳症・脊髄症の新たな展開 ヒトパピローマウイルスワクチ
1956
140.
1921
ン接種後の神経症状は、なぜ⼼因性疾患と間違われるのか.神経治療学 2018;35:536-542.
1957
Effectiveness Analysis of a Quadrivalent Human Papillomavirus Vaccine (6/11/16/18) for
1922
http://search.jamas.or.jp/link/ui/2019260843
1958
Females in Japan.Value Health Reg Issues. 2013;2(1):92-7.
1923
130. Ushida, T., et al. (2016). The Effect of Guidance regarding Home Exercise and ADL
1959
141.
1924
on Adolescent Females Suffering from Adverse Effects after HPV Vaccination in Japanese
1960
effectiveness of routine and catch-up vaccination of girls and women with a nine-valent HPV
1925
Multidisciplinary Pain Centers. Pain Res Manag 2016: 3689352.
1961
vaccine in Japan: a model-based study. BMC Infect Dis. 2021;21(1):11
1926
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4904598/pdf/PRM3682016-3689352.pdf
1962
142 . Isshiki T. HPV vaccination for cervical cancer prevention is not cost-effective in Japan.
1927
131. WHO (2017). Human papillomavirus vaccines: WHO position paper, May 2017. Wkly
1963
Asian Pac J Cancer Prev. 2014; 15(15): 6177-80.
1928
Epidemiol Rec 92(19): 241-268.
1964
143 . Kitano T. Stopping the HPV vaccine crisis in Japan: Quantifying the benefits and risks
1929
132. Centers for Disease Control and Prevention.(2020). Vaccine for HPV.
1965
of HPV vaccination in quality-adjusted life-years for appropriate decision-making. J Infect
1930
https://www.cdc.gov/hpv/parents/vaccinesafety.html
1966
Chemother. 2020; 26(3): 225-30.
1931
133. Control, E. C. f. D. P. a. (2020). Guidance on HPV vaccination in EU countries: focus
1967
144. 厚⽣労働省. 第 14 回厚⽣科学審議会予防接種・ワクチン分科会予防接種基本⽅針部
1932
on boys, people living with HIV and 9-valent HPV vaccine introduction.
1968
会 資料 3 沈降13価肺炎球菌結合型ワクチンを⾼齢者へ定期接種で使⽤することの是⾮
1933
https://www.ecdc.europa.eu/sites/default/files/documents/Guidance-on-HPV-vaccination
1969
に関する検討⽅針について
1934
-in-EU-countries2020-03-30.pdf
1970
https://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-
1935
134. Agency, E. M. HPV vaccines: EMA confirms evidence does not support that they cause
1971
Kouseikagakuka/0000111764.pdf
1936
CRPS or POTS 2016.
1972
145. Taguchi, A., et al. (2020). Multistate Markov Model to Predict the Prognosis of High-
1937
http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vacc
1973
Risk Human Papillomavirus-Related Cervical Lesions. Cancers (Basel) 12(2).
1938
ines_20/European_Commission_final_decision/WC500196773.pdf
1974
146. 村澤秀樹 ⼤久保⼀郎, 荒川⼀郎. (2014). ⼦宮頸がん検診へのヒトパピローマウイル
1939
135. Zhang, Z., et al. (2017). Expanded strain coverage for a highly successful public health
1975
ス DNA 検査導⼊に関する費⽤対効果分析.⽇本予防医学会雑誌 2014;9 (2):83-91.
1940
tool: Prophylactic 9-valent human papillomavirus vaccine. Hum Vaccin Immunother 13(10):
1976
147. De Vincenzo, R., et al. (2014). Long-term efficacy and safety of human papillomavirus
1941
2280-2291. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5647960/pdf/khvi-5647913-
1977
vaccination. Int J Womens Health 6: 999-1010.
1942
5647910-1346755.pdf
1978
148. Datta, S., et al. (2019). Assessing the cost-effectiveness of HPV vaccination strategies
1943
136. Yang, D. Y. and K. Bracken (2016). Update on the new 9-valent vaccine for human
1979
for adolescent girls and boys in the UK. BMC Infect Dis 19(1): 552.
1944
papillomavirus prevention. Can Fam Physician 62(5): 399-402.
1980
149. Brisson, M., et al. (2016). Health and Economic Impact of Switching from a 4-Valent
1945
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865336/pdf/0620399.pdf
1981
to a 9-Valent HPV Vaccination Program in the United States. J Natl Cancer Inst 108(1).
1946
137.
Luxembourg, A., et al. (2015). Phase II studies to select the formulation of a
1982
150. Murasawa, H., et al. (2014). Evaluation of health-related quality of life for hypothesized
1947
multivalent HPV L1 virus-like particle (VLP) vaccine. Hum Vaccin Immunother 11(6): 1313-
1983
medical states associated with cervical cancer. Asian Pac J Cancer Prev 15(22): 9679-9685.
91
Konno R, Sasagawa T, Fukuda T, Van Kriekinge G, Demarteau N. (2010).Cost-
Yamamoto N, Mori R, Jacklin P, Osuga Y, Kawana K, Shibuya K, et al.(2012).
Yamabe K, Singhal PK, Abe M, Dasbach EJ, Elbasha EH. (2013).The Cost-
Cody P, Tobe K, Abe M, Elbasha EH.(2012). Public health impact and cost
92