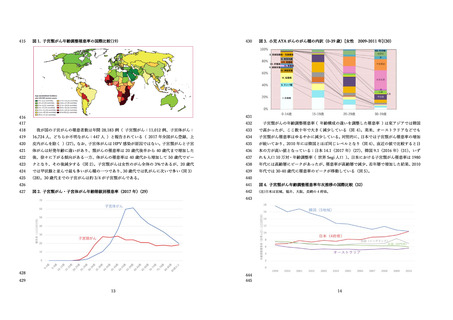
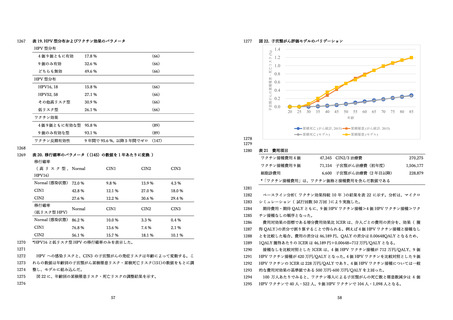
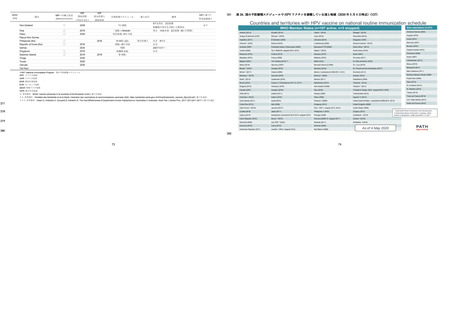
よむ、つかう、まなぶ。
11【参考資料1-6】9価HPVワクチンファクトシート (47 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29181.html |
| 出典情報 | 厚生科学審議会予防接種・ワクチン分科会(第41回 11/18)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
1984
151. 国⽴がん研究センター がん対策情報センター. (2020)がん情報サービス「がん登録・
2020
162. Fagot, J. P., et al. (2011). HPV vaccination in France: uptake, costs and issues for the
1985
統計」年齢階級別累積がん罹患リスク・累積がん死亡リスク (2017 年)
2021
National Health Insurance. Vaccine 29(19): 3610-3616.
1986
http://gdb.ganjoho.jp/graph_db/gdb1?smTypes=17
2022
https://www.ncbi.nlm.nih.gov/pubmed/21382486
1987
152. WHO. (2020). Immunization, Vaccines and Biologicals_ Data, statistics and graphics.
2023
163. Hequet, D., et al. (2015). Age impact on human papillomavirus vaccination in France
1988
6. Immunization schedule. Data are available for: 6.6 Slides on introduction status of selected
2024
in 2014: A study from the National Health Insurance Database. Bull Cancer 102(11): 892-
1989
vaccines.
2025
897. https://www.ncbi.nlm.nih.gov/pubmed/26526386
1990
https://www.who.int/teams/immunization-vaccines-and-biologicals/data-statistics-and-
2026
164.
1991
graphics
2027
recommandations vaccinales 2020.
1992
153. WHO. (2019). GLOBAL MARKET STUDY HPV.
2028
https://solidarites-sante.gouv.fr/IMG/pdf/calendrier_vaccinal_29juin20.pdf
1993
https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/m
2029
165. Lefèvre, H., et al. (2018). The New HPV Vaccination Policy in France. N Engl J Med
1994
odule2/WHO_HPV_market_study_public_summary_Dec2019.pdf
2030
378(12): 1160.
1995
154. Bloem, P. (2019). Update on access to HPV vaccine. SAGE meeting of October 2019.
2031
166. Lefèvre, H., et al. (2018). HPV vaccination rate in French adolescent girls: an example
1996
https://www.who.int/immunization/sage/meetings/2019/october/bloem_hpv_sage_octobe
2032
of vaccine distrust. Arch Dis Child 103(8): 740-746.
1997
r_2019.pdf
2033
167. Cappelli, M. G., et al. (2018). Cervical cancer prevention: An Italian scenario between
1998
155.
2034
organised screening and human papillomaviruses vaccination. Eur J Cancer Care (Engl)
1999
summary. https://apps.who.int/immunization_monitoring/globalsummary
2035
27(5): e12905.
2000
156. ECDC Vaccine schedules in all countries of the European Union.
2036
168. Acampora A, G. A., Barbara A, Causio A, Calabrò G.E., Cicchetti A., Waure Cd (2019).
2001
https://vaccine-schedule.ecdc.europa.eu/
2037
Strategies to achieve HPV-related disease control in Italy: results from an integrative
2002
157. PATH. (2020). Global HPV vaccine introduction review. .
2038
approach. Epidemiology Biostatistics and Public Health. 2019;16(3).
2003
https://path.azureedge.net/media/documents/Global_HPV_Vaccine_Intro_Overview_Slide
2039
https://academic.oup.com/eurpub/article/29/Supplement_4/ckz186.511/5623409
2004
s_webversion_2020May.pdf
2040
169. GOV.UK . (2019). HPV universal vaccination guidance for healthcare practitioners.
2005
158. WHO .(2019). Monitoring and Surveillance of HPV Vaccination Programmes. WHO
2041
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_
2006
estimates of Human papillomavirus immunization coverage 2010-2018
2042
data/file/813014/PHE_HPV_universal_programme_guidance.pdf
2007
https://www.who.int/immunization/monitoring_surveillance/data/HPV_estimates.xls?ua=
2043
170. GOV.UK . (2019). Greenbook_chapter_18a.
2008
1
2044
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_
2009
159. ECDC. (2019). Public consultation on draft guidance for introduction of HPV vaccines
2045
data/file/828868/Greenbook_chapter_18a.pdf
2010
in EU countries: focus on 9-valent HPV vaccine and vaccination of boys and people living
2046
171. U.S. Food & Drug Administration. (2020). Gardasil 9. https://www.fda.gov/vaccines-
2011
with HIV.
2047
blood-biologics/vaccines/gardasil-9
2012
https://www.ecdc.europa.eu/sites/portal/files/documents/hpv-public-consultation-1-April-
2048
172. Centers for Disease Control and Prevention.Vaccine Information Statements (VISs)
2013
2019.pdf
2049
What's New with VISs. https://www.cdc.gov/vaccines/hcp/vis/what-is-new.html
2014
160.
2050
173.
2015
https://www.ema.europa.eu/en/documents/overview/gardasil-9-epar-summary-
2051
https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
WHO. (2019).
vaccine-preventable diseases: monitoring system. 2019 global
European Medicine Agency. (2016). EPAR summary for the public. Gardasil 9.
Ministère des Solidarités et de la Santé.
(2020).
Calendrier des vaccinations et
Centers for Disease Control and Prevention.(2020). Immunization Schedules.
2016
public_en.pdf
2052
174. U.S. Food and Drug Administration. (2020). Package Insert - GARDASIL 9 ‒ FDA.
2017
161. Buttmann-Schweiger, N., et al. (2017). Cancer incidence in Germany attributable to
2053
https://www.fda.gov/media/90064/download
2018
human papillomavirus in 2013. BMC Cancer 17(1): 682.
2054
175.
2019
https://www.ncbi.nlm.nih.gov/pubmed/29037233
2055
https://www.fda.gov/media/138949/download
93
U.S. Food and Drug Administration. (2020). Supplement Acceletrated Approval.
94
151. 国⽴がん研究センター がん対策情報センター. (2020)がん情報サービス「がん登録・
2020
162. Fagot, J. P., et al. (2011). HPV vaccination in France: uptake, costs and issues for the
1985
統計」年齢階級別累積がん罹患リスク・累積がん死亡リスク (2017 年)
2021
National Health Insurance. Vaccine 29(19): 3610-3616.
1986
http://gdb.ganjoho.jp/graph_db/gdb1?smTypes=17
2022
https://www.ncbi.nlm.nih.gov/pubmed/21382486
1987
152. WHO. (2020). Immunization, Vaccines and Biologicals_ Data, statistics and graphics.
2023
163. Hequet, D., et al. (2015). Age impact on human papillomavirus vaccination in France
1988
6. Immunization schedule. Data are available for: 6.6 Slides on introduction status of selected
2024
in 2014: A study from the National Health Insurance Database. Bull Cancer 102(11): 892-
1989
vaccines.
2025
897. https://www.ncbi.nlm.nih.gov/pubmed/26526386
1990
https://www.who.int/teams/immunization-vaccines-and-biologicals/data-statistics-and-
2026
164.
1991
graphics
2027
recommandations vaccinales 2020.
1992
153. WHO. (2019). GLOBAL MARKET STUDY HPV.
2028
https://solidarites-sante.gouv.fr/IMG/pdf/calendrier_vaccinal_29juin20.pdf
1993
https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/m
2029
165. Lefèvre, H., et al. (2018). The New HPV Vaccination Policy in France. N Engl J Med
1994
odule2/WHO_HPV_market_study_public_summary_Dec2019.pdf
2030
378(12): 1160.
1995
154. Bloem, P. (2019). Update on access to HPV vaccine. SAGE meeting of October 2019.
2031
166. Lefèvre, H., et al. (2018). HPV vaccination rate in French adolescent girls: an example
1996
https://www.who.int/immunization/sage/meetings/2019/october/bloem_hpv_sage_octobe
2032
of vaccine distrust. Arch Dis Child 103(8): 740-746.
1997
r_2019.pdf
2033
167. Cappelli, M. G., et al. (2018). Cervical cancer prevention: An Italian scenario between
1998
155.
2034
organised screening and human papillomaviruses vaccination. Eur J Cancer Care (Engl)
1999
summary. https://apps.who.int/immunization_monitoring/globalsummary
2035
27(5): e12905.
2000
156. ECDC Vaccine schedules in all countries of the European Union.
2036
168. Acampora A, G. A., Barbara A, Causio A, Calabrò G.E., Cicchetti A., Waure Cd (2019).
2001
https://vaccine-schedule.ecdc.europa.eu/
2037
Strategies to achieve HPV-related disease control in Italy: results from an integrative
2002
157. PATH. (2020). Global HPV vaccine introduction review. .
2038
approach. Epidemiology Biostatistics and Public Health. 2019;16(3).
2003
https://path.azureedge.net/media/documents/Global_HPV_Vaccine_Intro_Overview_Slide
2039
https://academic.oup.com/eurpub/article/29/Supplement_4/ckz186.511/5623409
2004
s_webversion_2020May.pdf
2040
169. GOV.UK . (2019). HPV universal vaccination guidance for healthcare practitioners.
2005
158. WHO .(2019). Monitoring and Surveillance of HPV Vaccination Programmes. WHO
2041
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_
2006
estimates of Human papillomavirus immunization coverage 2010-2018
2042
data/file/813014/PHE_HPV_universal_programme_guidance.pdf
2007
https://www.who.int/immunization/monitoring_surveillance/data/HPV_estimates.xls?ua=
2043
170. GOV.UK . (2019). Greenbook_chapter_18a.
2008
1
2044
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_
2009
159. ECDC. (2019). Public consultation on draft guidance for introduction of HPV vaccines
2045
data/file/828868/Greenbook_chapter_18a.pdf
2010
in EU countries: focus on 9-valent HPV vaccine and vaccination of boys and people living
2046
171. U.S. Food & Drug Administration. (2020). Gardasil 9. https://www.fda.gov/vaccines-
2011
with HIV.
2047
blood-biologics/vaccines/gardasil-9
2012
https://www.ecdc.europa.eu/sites/portal/files/documents/hpv-public-consultation-1-April-
2048
172. Centers for Disease Control and Prevention.Vaccine Information Statements (VISs)
2013
2019.pdf
2049
What's New with VISs. https://www.cdc.gov/vaccines/hcp/vis/what-is-new.html
2014
160.
2050
173.
2015
https://www.ema.europa.eu/en/documents/overview/gardasil-9-epar-summary-
2051
https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
WHO. (2019).
vaccine-preventable diseases: monitoring system. 2019 global
European Medicine Agency. (2016). EPAR summary for the public. Gardasil 9.
Ministère des Solidarités et de la Santé.
(2020).
Calendrier des vaccinations et
Centers for Disease Control and Prevention.(2020). Immunization Schedules.
2016
public_en.pdf
2052
174. U.S. Food and Drug Administration. (2020). Package Insert - GARDASIL 9 ‒ FDA.
2017
161. Buttmann-Schweiger, N., et al. (2017). Cancer incidence in Germany attributable to
2053
https://www.fda.gov/media/90064/download
2018
human papillomavirus in 2013. BMC Cancer 17(1): 682.
2054
175.
2019
https://www.ncbi.nlm.nih.gov/pubmed/29037233
2055
https://www.fda.gov/media/138949/download
93
U.S. Food and Drug Administration. (2020). Supplement Acceletrated Approval.
94