よむ、つかう、まなぶ。
資料1-2 調査結果報告書 (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27607.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第10回) |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
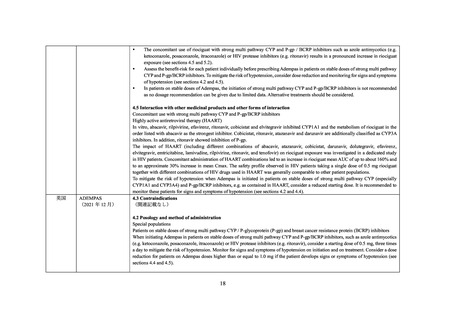
In vitro, rilpivirine, abacavir, efavirenz, ritonavir, cobicistat and elvitegravir inhibited CYP1A1 and the metabolism of riociguat in the
order listed with rilpivirine as the strongest inhibitor. Cobicistat, ritonavir, atazanavir and darunavir are additionally classified as CYP3A
inhibitors. In addition, ritonavir showed inhibition of P-gp.
The impact of HAART (including different combinations of abacavir, atazanavir, cobicistat, darunavir, dolutegravir, efavirenz,
elvitegravir, emtricitabine, lamivudine, rilpivirine, ritonavir, and tenofovir) on riociguat exposure was investigated in a dedicated study
in HIV patients. Concomitant administration of HAART combinations led to an increase in riociguat mean AUC of up to about 160% and
up to an approximate 29% increase in mean Cmax. The safety profile observed in HIV patients taking a single dose of 0.5 mg riociguat
together with different combinations of HIV drugs used in HAART was generally comparable to other patient populations.
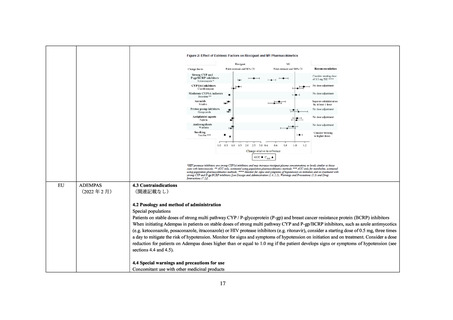
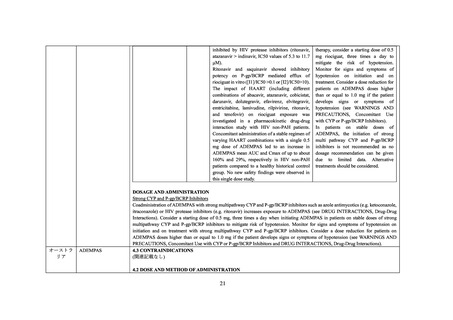
When initiating ADEMPAS treatment in patients on stable doses of strong multi pathway CYP and P-gp/BCRP inhibitors, e.g. as
contained in HAART therapy, consider a starting dose of 0.5 mg riociguat, three times a day to mitigate the risk of hypotension. Monitor
for signs and symptoms of hypotension on initiation and on treatment. Consider a dose reduction for patients on ADEMPAS doses higher
than or equal to 1.0 mg if the patient develops signs or symptoms of hypotension (see Sections 4.2 DOSE AND METHOD OF
ADMINISTRATION, 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE and 5.2 PHARMACOKINETIC PROPERTIES).
In patients on stable doses of ADEMPAS, the initiation of strong multi pathway CYP and P-gp/BCRP inhibitors is not recommended as
no dosage recommendation can be given due to limited data. Alternative treatments should be considered.
23
order listed with rilpivirine as the strongest inhibitor. Cobicistat, ritonavir, atazanavir and darunavir are additionally classified as CYP3A
inhibitors. In addition, ritonavir showed inhibition of P-gp.
The impact of HAART (including different combinations of abacavir, atazanavir, cobicistat, darunavir, dolutegravir, efavirenz,
elvitegravir, emtricitabine, lamivudine, rilpivirine, ritonavir, and tenofovir) on riociguat exposure was investigated in a dedicated study
in HIV patients. Concomitant administration of HAART combinations led to an increase in riociguat mean AUC of up to about 160% and
up to an approximate 29% increase in mean Cmax. The safety profile observed in HIV patients taking a single dose of 0.5 mg riociguat
together with different combinations of HIV drugs used in HAART was generally comparable to other patient populations.
When initiating ADEMPAS treatment in patients on stable doses of strong multi pathway CYP and P-gp/BCRP inhibitors, e.g. as
contained in HAART therapy, consider a starting dose of 0.5 mg riociguat, three times a day to mitigate the risk of hypotension. Monitor
for signs and symptoms of hypotension on initiation and on treatment. Consider a dose reduction for patients on ADEMPAS doses higher
than or equal to 1.0 mg if the patient develops signs or symptoms of hypotension (see Sections 4.2 DOSE AND METHOD OF
ADMINISTRATION, 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE and 5.2 PHARMACOKINETIC PROPERTIES).
In patients on stable doses of ADEMPAS, the initiation of strong multi pathway CYP and P-gp/BCRP inhibitors is not recommended as
no dosage recommendation can be given due to limited data. Alternative treatments should be considered.
23