よむ、つかう、まなぶ。
資料1-2 調査結果報告書 (24 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27607.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第10回) |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
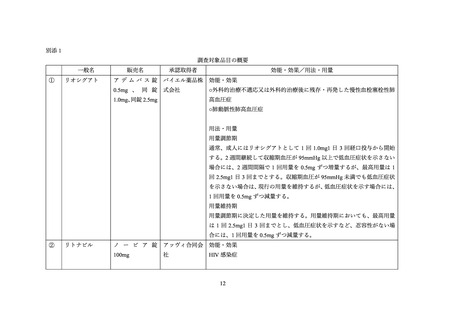
表 2:リトナビル含有製剤の海外添付文書におけるリオシグアトとの併用についての関連記載
国・地域
リトナビル
米国
EU
製品名
(添付文書の版)
NORVIR
(2020 年 10 月)
NORVIR
(2021 年 3 月)
記載状況
(関連記載なし)
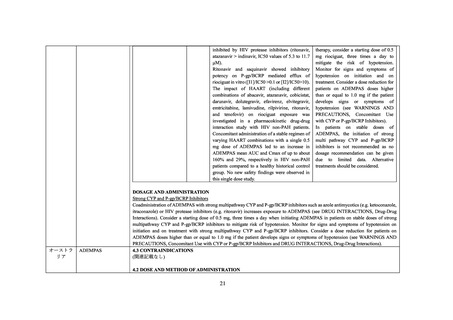
4.3 Contraindications
(関連記載なし)
4.4 Special warnings and precautions for use
Interactions with other medicinal products
Riociguat
The concomitant use of ritonavir is not recommended due to potential increase in riociguat exposure (see section 4.5).
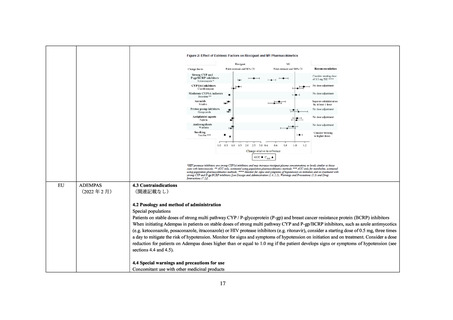
4.5 Interaction with other medicinal products and other forms of interaction
Medicinal product that are affected by the use of ritonavir
Riociguat
Serum concentrations may be increased due to CYP3A and P-gp inhibition by ritonavir. The co-administration of riociguat with Norvir
is not recommended (see section 4.4 and refer to riociguat SmPC).
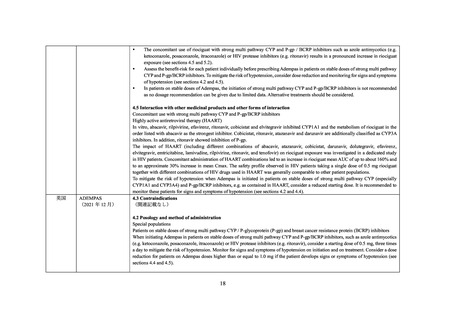
英国
NORVIR
(2021 年 1 月)
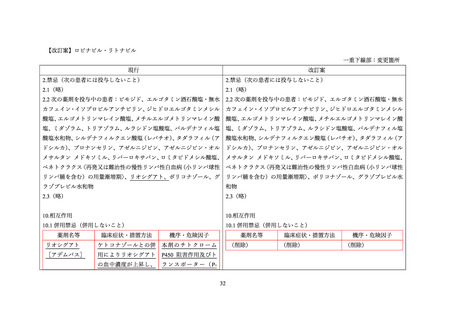
4.3 Contraindications
(関連記載なし)
4.4 Special warnings and precautions for use
Interactions with other medicinal products
Riociguat
The concomitant use of ritonavir is not recommended due to potential increase in riociguat exposure (see section 4.5).
4.5 Interaction with other medicinal products and other forms of interaction
Medicinal product that are affected by the use of ritonavir
Riociguat
Serum concentrations may be increased due to CYP3A and P-gp inhibition by ritonavir. The co-administration of riociguat with Norvir
is not recommended (see section 4.4 and refer to riociguat SmPC).
24
国・地域
リトナビル
米国
EU
製品名
(添付文書の版)
NORVIR
(2020 年 10 月)
NORVIR
(2021 年 3 月)
記載状況
(関連記載なし)
4.3 Contraindications
(関連記載なし)
4.4 Special warnings and precautions for use
Interactions with other medicinal products
Riociguat
The concomitant use of ritonavir is not recommended due to potential increase in riociguat exposure (see section 4.5).
4.5 Interaction with other medicinal products and other forms of interaction
Medicinal product that are affected by the use of ritonavir
Riociguat
Serum concentrations may be increased due to CYP3A and P-gp inhibition by ritonavir. The co-administration of riociguat with Norvir
is not recommended (see section 4.4 and refer to riociguat SmPC).
英国
NORVIR
(2021 年 1 月)
4.3 Contraindications
(関連記載なし)
4.4 Special warnings and precautions for use
Interactions with other medicinal products
Riociguat
The concomitant use of ritonavir is not recommended due to potential increase in riociguat exposure (see section 4.5).
4.5 Interaction with other medicinal products and other forms of interaction
Medicinal product that are affected by the use of ritonavir
Riociguat
Serum concentrations may be increased due to CYP3A and P-gp inhibition by ritonavir. The co-administration of riociguat with Norvir
is not recommended (see section 4.4 and refer to riociguat SmPC).
24