よむ、つかう、まなぶ。
資料2-1 一般社団法人次世代基盤政策研究所 御提出資料 (19 ページ)
出典
| 公開元URL | https://www8.cao.go.jp/kisei-kaikaku/kisei/meeting/wg/2409_04medical/241125/medical03_agenda.html |
| 出典情報 | 規制改革推進会議 健康・医療・介護ワーキング・グループ(第3回 11/25)《内閣府》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
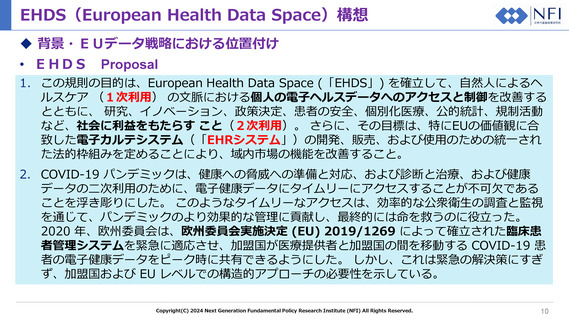
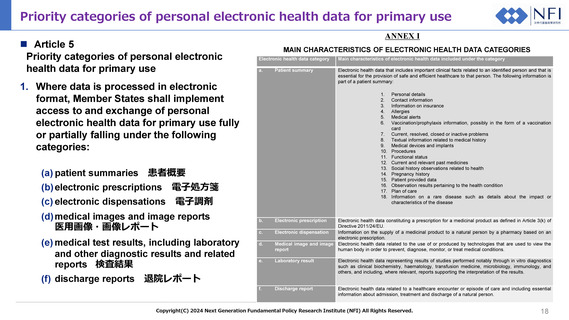
Priority categories of personal electronic health data for primary use
Article 5
Priority categories of personal electronic
health data for primary use
ANNEX I
MAIN CHARACTERISTICS OF ELECTRONIC HEALTH DATA CATEGORIES
Electronic health data category
Main characteristics of electronic health data included under the category
a.
Electronic health data that includes important clinical facts related to an identified person and that is
essential for the provision of safe and efficient healthcare to that person. The following information is
part of a patient summary:
Patient summary
1. Where data is processed in electronic
format, Member States shall implement
access to and exchange of personal
electronic health data for primary use fully
or partially falling under the following
categories:
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
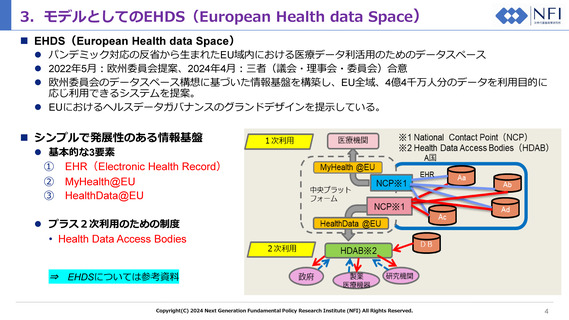
(a) patient summaries 患者概要
(b) electronic prescriptions
電子処方箋
(c) electronic dispensations
電子調剤
(d) medical images and image reports
医用画像・画像レポート
(e) medical test results, including laboratory
and other diagnostic results and related
reports 検査結果
(f) discharge reports 退院レポート
b.
c.
d.
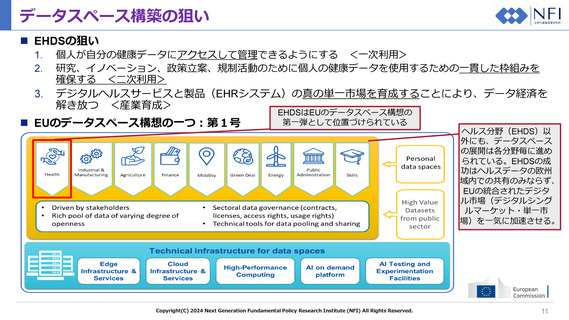
Personal details
Contact information
Information on insurance
Allergies
Medical alerts
Vaccination/prophylaxis information, possibly in the form of a vaccination
card
Current, resolved, closed or inactive problems
Textual information related to medical history
Medical devices and implants
Procedures
Functional status
Current and relevant past medicines
Social history observations related to health
Pregnancy history
Patient provided data
Observation results pertaining to the health condition
Plan of care
Information on a rare disease such as details about the impact or
characteristics of the disease
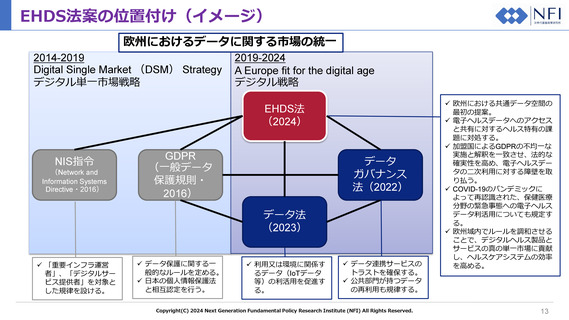
Electronic prescription
Electronic health data constituting a prescription for a medicinal product as defined in Article 3(k) of
Directive 2011/24/EU.
Electronic dispensation
Information on the supply of a medicinal product to a natural person by a pharmacy based on an
electronic prescription.
Medical image and image Electronic health data related to the use of or produced by technologies that are used to view the
report
human body in order to prevent, diagnose, monitor, or treat medical conditions.
e.
Laboratory result
Electronic health data representing results of studies performed notably through in vitro diagnostics
such as clinical biochemistry, haematology, transfusion medicine, microbiology, immunology, and
others, and including, where relevant, reports supporting the interpretation of the results.
f.
Discharge report
Electronic health data related to a healthcare encounter or episode of care and including essential
information about admission, treatment and discharge of a natural person.
Copyright(C) 2024 Next Generation Fundamental Policy Research Institute (NFI) All Rights Reserved.
18
Article 5
Priority categories of personal electronic
health data for primary use
ANNEX I
MAIN CHARACTERISTICS OF ELECTRONIC HEALTH DATA CATEGORIES
Electronic health data category
Main characteristics of electronic health data included under the category
a.
Electronic health data that includes important clinical facts related to an identified person and that is
essential for the provision of safe and efficient healthcare to that person. The following information is
part of a patient summary:
Patient summary
1. Where data is processed in electronic
format, Member States shall implement
access to and exchange of personal
electronic health data for primary use fully
or partially falling under the following
categories:
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
(a) patient summaries 患者概要
(b) electronic prescriptions
電子処方箋
(c) electronic dispensations
電子調剤
(d) medical images and image reports
医用画像・画像レポート
(e) medical test results, including laboratory
and other diagnostic results and related
reports 検査結果
(f) discharge reports 退院レポート
b.
c.
d.
Personal details
Contact information
Information on insurance
Allergies
Medical alerts
Vaccination/prophylaxis information, possibly in the form of a vaccination
card
Current, resolved, closed or inactive problems
Textual information related to medical history
Medical devices and implants
Procedures
Functional status
Current and relevant past medicines
Social history observations related to health
Pregnancy history
Patient provided data
Observation results pertaining to the health condition
Plan of care
Information on a rare disease such as details about the impact or
characteristics of the disease
Electronic prescription
Electronic health data constituting a prescription for a medicinal product as defined in Article 3(k) of
Directive 2011/24/EU.
Electronic dispensation
Information on the supply of a medicinal product to a natural person by a pharmacy based on an
electronic prescription.
Medical image and image Electronic health data related to the use of or produced by technologies that are used to view the
report
human body in order to prevent, diagnose, monitor, or treat medical conditions.
e.
Laboratory result
Electronic health data representing results of studies performed notably through in vitro diagnostics
such as clinical biochemistry, haematology, transfusion medicine, microbiology, immunology, and
others, and including, where relevant, reports supporting the interpretation of the results.
f.
Discharge report
Electronic health data related to a healthcare encounter or episode of care and including essential
information about admission, treatment and discharge of a natural person.
Copyright(C) 2024 Next Generation Fundamental Policy Research Institute (NFI) All Rights Reserved.
18