よむ、つかう、まなぶ。
資料2-1 一般社団法人次世代基盤政策研究所 御提出資料 (22 ページ)
出典
| 公開元URL | https://www8.cao.go.jp/kisei-kaikaku/kisei/meeting/wg/2409_04medical/241125/medical03_agenda.html |
| 出典情報 | 規制改革推進会議 健康・医療・介護ワーキング・グループ(第3回 11/25)《内閣府》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
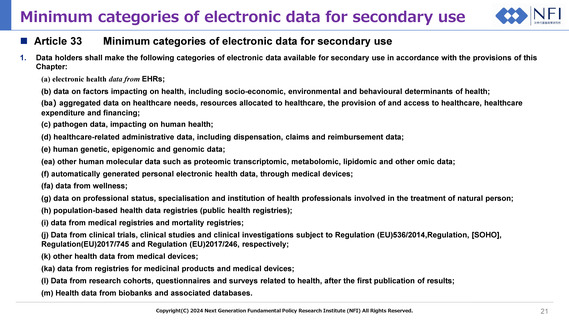
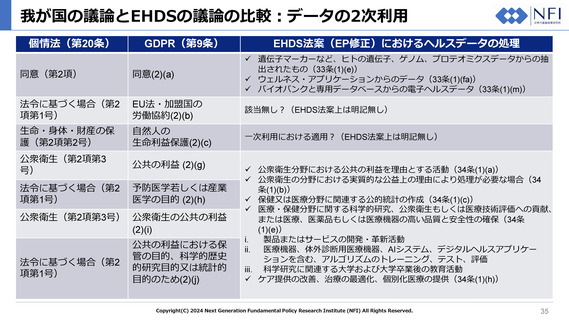
Minimum categories of electronic data for secondary use
Article 33
1.
Minimum categories of electronic data for secondary use
Data holders shall make the following categories of electronic data available for secondary use in accordance with the provisions of this
Chapter:
(a) electronic health data from EHRs;
(b) data on factors impacting on health, including socio-economic, environmental and behavioural determinants of health;
(ba) aggregated data on healthcare needs, resources allocated to healthcare, the provision of and access to healthcare, healthcare
expenditure and financing;
(c) pathogen data, impacting on human health;
(d) healthcare-related administrative data, including dispensation, claims and reimbursement data;
(e) human genetic, epigenomic and genomic data;
(ea) other human molecular data such as proteomic transcriptomic, metabolomic, lipidomic and other omic data;
(f) automatically generated personal electronic health data, through medical devices;
(fa) data from wellness;
(g) data on professional status, specialisation and institution of health professionals involved in the treatment of natural person;
(h) population-based health data registries (public health registries);
(i) data from medical registries and mortality registries;
(j) Data from clinical trials, clinical studies and clinical investigations subject to Regulation (EU)536/2014,Regulation, [SOHO],
Regulation(EU)2017/745 and Regulation (EU)2017/246, respectively;
(k) other health data from medical devices;
(ka) data from registries for medicinal products and medical devices;
(l) Data from research cohorts, questionnaires and surveys related to health, after the first publication of results;
(m) Health data from biobanks and associated databases.
Copyright(C) 2024 Next Generation Fundamental Policy Research Institute (NFI) All Rights Reserved.
21
Article 33
1.
Minimum categories of electronic data for secondary use
Data holders shall make the following categories of electronic data available for secondary use in accordance with the provisions of this
Chapter:
(a) electronic health data from EHRs;
(b) data on factors impacting on health, including socio-economic, environmental and behavioural determinants of health;
(ba) aggregated data on healthcare needs, resources allocated to healthcare, the provision of and access to healthcare, healthcare
expenditure and financing;
(c) pathogen data, impacting on human health;
(d) healthcare-related administrative data, including dispensation, claims and reimbursement data;
(e) human genetic, epigenomic and genomic data;
(ea) other human molecular data such as proteomic transcriptomic, metabolomic, lipidomic and other omic data;
(f) automatically generated personal electronic health data, through medical devices;
(fa) data from wellness;
(g) data on professional status, specialisation and institution of health professionals involved in the treatment of natural person;
(h) population-based health data registries (public health registries);
(i) data from medical registries and mortality registries;
(j) Data from clinical trials, clinical studies and clinical investigations subject to Regulation (EU)536/2014,Regulation, [SOHO],
Regulation(EU)2017/745 and Regulation (EU)2017/246, respectively;
(k) other health data from medical devices;
(ka) data from registries for medicinal products and medical devices;
(l) Data from research cohorts, questionnaires and surveys related to health, after the first publication of results;
(m) Health data from biobanks and associated databases.
Copyright(C) 2024 Next Generation Fundamental Policy Research Institute (NFI) All Rights Reserved.
21