よむ、つかう、まなぶ。
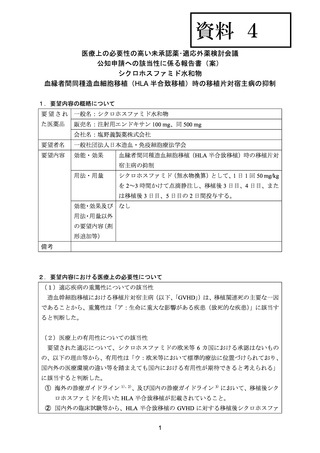
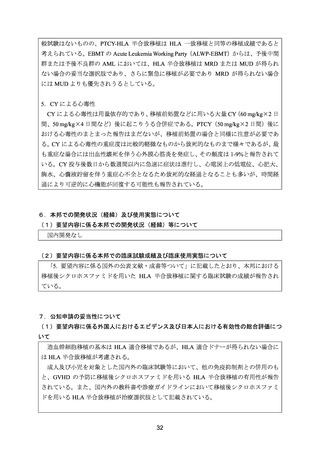
資料4 シクロホスファミド水和物 (38 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00027.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第55回 5/31)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
504.
31) Ayas M, et. al. Successful outcome in patients with Fanconi anemia undergoing T cellreplete mismatched related donor hematopoietic cell transplantation using reduced-dose
cyclophosphamide post-transplantation. Biol Blood Marrow Transplant 2019; 25: 2217-21.
32) Trujillo ÁM, et al. Haploidentical hematopoietic stem cell transplantation with posttransplantation cyclophosphamide in children with high-risk leukemia using a reduced-intensity
conditioning regimen and peripheral blood as the stem cell source. Transplant Cell Ther 2021;
27: 427.e1-7.
33) Pérez-Martínez A, et al. Haploidentical transplantation in high-risk pediatric leukemia: A
retrospective comparative analysis on behalf of the Spanish working Group for bone marrow
transplantation in children (GETMON) and the Spanish Grupo for hematopoietic transplantation
(GETH). Am J Hematol 2020; 95: 28-37.
34) Uygun V, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant highdose cyclophosphamide in high-risk children: A single-center study. Pediatr Transplant 2019;
23: e13546.
35) Hong KT, et al. Favorable outcome of post-transplantation cyclophosphamide haploidentical
peripheral blood stem cell transplantation with targeted busulfan-based myeloablative
conditioning using intensive pharmacokinetic monitoring in pediatric patients. Biol Blood
Marrow Transplant 2018; 24: 2239-44.
36) Klein OR, et al. Nonmyeloablative haploidentical bone marrow transplantation with posttransplantation cyclophosphamide for pediatric and young adult patients with high-risk
hematologic malignancies. Biol Blood Marrow Transplant 2017; 23: 325-32.
37) Berger M, et al. Feasibility and outcome of haploidentical hematopoietic stem cell
transplantation with post-transplant high-dose cyclophosphamide for children and adolescents
with hematologic malignancies: An AIEOP-GITMO retrospective multicenter study. Biol Blood
Marrow Transplant 2016; 22: 902-9.
38) Yesilipek MA, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant
high-dose cyclophosphamide in high-risk children: A single-center study. Pediatr Transplant
2016; 20: 417-23.
39) Fernandes J F, et. al. Outcomes after haploidentical stem cell transplantation with posttransplantation cyclophosphamide in patients with primary immunodeficiency diseases. Biol
Blood Marrow Transplant 2020; 26: 1923-9.
40) Rocha V, et. al. Impact of mother donor, peripheral blood stem cells and measurable residual
disease on outcomes after haploidentical hematopoietic cell transplantation with post-transplant
cyclophosphamide in children with acute leukaemia. Bone Marrow Transplant 2021; 56: 30428.
41) Neven B, et. al. Haploidentical hematopoietic stem cell transplantation with post-transplant
38
31) Ayas M, et. al. Successful outcome in patients with Fanconi anemia undergoing T cellreplete mismatched related donor hematopoietic cell transplantation using reduced-dose
cyclophosphamide post-transplantation. Biol Blood Marrow Transplant 2019; 25: 2217-21.
32) Trujillo ÁM, et al. Haploidentical hematopoietic stem cell transplantation with posttransplantation cyclophosphamide in children with high-risk leukemia using a reduced-intensity
conditioning regimen and peripheral blood as the stem cell source. Transplant Cell Ther 2021;
27: 427.e1-7.
33) Pérez-Martínez A, et al. Haploidentical transplantation in high-risk pediatric leukemia: A
retrospective comparative analysis on behalf of the Spanish working Group for bone marrow
transplantation in children (GETMON) and the Spanish Grupo for hematopoietic transplantation
(GETH). Am J Hematol 2020; 95: 28-37.
34) Uygun V, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant highdose cyclophosphamide in high-risk children: A single-center study. Pediatr Transplant 2019;
23: e13546.
35) Hong KT, et al. Favorable outcome of post-transplantation cyclophosphamide haploidentical
peripheral blood stem cell transplantation with targeted busulfan-based myeloablative
conditioning using intensive pharmacokinetic monitoring in pediatric patients. Biol Blood
Marrow Transplant 2018; 24: 2239-44.
36) Klein OR, et al. Nonmyeloablative haploidentical bone marrow transplantation with posttransplantation cyclophosphamide for pediatric and young adult patients with high-risk
hematologic malignancies. Biol Blood Marrow Transplant 2017; 23: 325-32.
37) Berger M, et al. Feasibility and outcome of haploidentical hematopoietic stem cell
transplantation with post-transplant high-dose cyclophosphamide for children and adolescents
with hematologic malignancies: An AIEOP-GITMO retrospective multicenter study. Biol Blood
Marrow Transplant 2016; 22: 902-9.
38) Yesilipek MA, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant
high-dose cyclophosphamide in high-risk children: A single-center study. Pediatr Transplant
2016; 20: 417-23.
39) Fernandes J F, et. al. Outcomes after haploidentical stem cell transplantation with posttransplantation cyclophosphamide in patients with primary immunodeficiency diseases. Biol
Blood Marrow Transplant 2020; 26: 1923-9.
40) Rocha V, et. al. Impact of mother donor, peripheral blood stem cells and measurable residual
disease on outcomes after haploidentical hematopoietic cell transplantation with post-transplant
cyclophosphamide in children with acute leukaemia. Bone Marrow Transplant 2021; 56: 30428.
41) Neven B, et. al. Haploidentical hematopoietic stem cell transplantation with post-transplant
38