よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (50 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
3) Companion animals
Source: Japanese Veterinary Antimicrobial Resistance Monitoring System (JVARM)
Routine monitoring of antimicrobial resistance in bacteria derived from diseased dogs and cats was launched in
FY2017, as part of efforts to strengthen monitoring under the AMR Action Plan. Monitoring of antimicrobial
resistance in bacteria derived from diseased animals, as opposed to those from healthy animals, has the potential
to be affected by the use of antimicrobials in treatment or by the incidence of diseases. As with food-producing
animals, obtaining information about antimicrobial resistance trends in healthy companion animals to serve as a
baseline is considered important. Accordingly, as well as ongoing monitoring of diseased animals, surveillance of
healthy dogs and cats was launched in 2018.
Antimicrobial susceptibility tests measured the MIC values of antimicrobials in respect of the bacterial strains
collected, using a broth microdilution method compliant with the CLSI Criteria. For agents with a BP indicated
by the CLSI, susceptibility was interpreted using the CLSI Criteria. The BPs of the other antimicrobial agents used
EUCAST values or were determined microbiologically (midpoint of a bimodal MIC distribution).
a. Bacterial strains from diseased dogs and cats
Bacterial strains from diseased dogs and cats were collected from small-animal clinical laboratories. The country
was divided into six regional blocks—Hokkaido and Tohoku, Kanto, Chubu, Kinki, Chugoku and Shikoku, and
Kyushu and Okinawa—and the number of strains allocated on the basis of the number of notifications of veterinary
clinic (small animal and other animals) establishment received.
Samples of Escherichia coli and Klebsiella spp. were collected from urine and reproductive organs, samples of
coagulase-positive Staphylococcus spp. from urine and skin, and samples of Enterococcus spp. from urine and
ears.
ⅰ. Escherichia coli
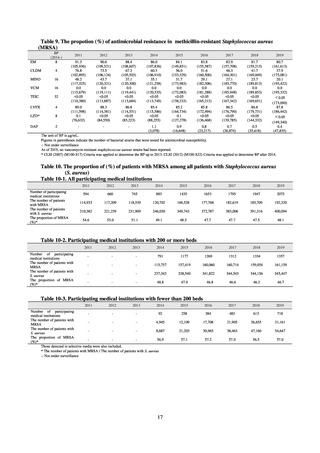
Monitoring of 14 agents was carried out for strains from dogs and cats. As in 2017 and 2018, rates of resistance
to ABPC and NA were high in 2019 at 46.9-60.2%. On the other hand, the rate of resistance to GM, KM, and CP
in strains isolated from dogs and cats was less than 20%. The rates of resistance to critically important
antimicrobials for human medicine in dog- and cat-derived strains respectively were as follows: 26.4-26.6% to
CTX; and 37.5-38.8% to CPFX. No resistance to CL and MEPM was observed.
49
Source: Japanese Veterinary Antimicrobial Resistance Monitoring System (JVARM)
Routine monitoring of antimicrobial resistance in bacteria derived from diseased dogs and cats was launched in
FY2017, as part of efforts to strengthen monitoring under the AMR Action Plan. Monitoring of antimicrobial
resistance in bacteria derived from diseased animals, as opposed to those from healthy animals, has the potential
to be affected by the use of antimicrobials in treatment or by the incidence of diseases. As with food-producing
animals, obtaining information about antimicrobial resistance trends in healthy companion animals to serve as a
baseline is considered important. Accordingly, as well as ongoing monitoring of diseased animals, surveillance of
healthy dogs and cats was launched in 2018.
Antimicrobial susceptibility tests measured the MIC values of antimicrobials in respect of the bacterial strains
collected, using a broth microdilution method compliant with the CLSI Criteria. For agents with a BP indicated
by the CLSI, susceptibility was interpreted using the CLSI Criteria. The BPs of the other antimicrobial agents used
EUCAST values or were determined microbiologically (midpoint of a bimodal MIC distribution).
a. Bacterial strains from diseased dogs and cats
Bacterial strains from diseased dogs and cats were collected from small-animal clinical laboratories. The country
was divided into six regional blocks—Hokkaido and Tohoku, Kanto, Chubu, Kinki, Chugoku and Shikoku, and
Kyushu and Okinawa—and the number of strains allocated on the basis of the number of notifications of veterinary
clinic (small animal and other animals) establishment received.
Samples of Escherichia coli and Klebsiella spp. were collected from urine and reproductive organs, samples of
coagulase-positive Staphylococcus spp. from urine and skin, and samples of Enterococcus spp. from urine and
ears.
ⅰ. Escherichia coli
Monitoring of 14 agents was carried out for strains from dogs and cats. As in 2017 and 2018, rates of resistance
to ABPC and NA were high in 2019 at 46.9-60.2%. On the other hand, the rate of resistance to GM, KM, and CP
in strains isolated from dogs and cats was less than 20%. The rates of resistance to critically important
antimicrobials for human medicine in dog- and cat-derived strains respectively were as follows: 26.4-26.6% to
CTX; and 37.5-38.8% to CPFX. No resistance to CL and MEPM was observed.
49