【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (95 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
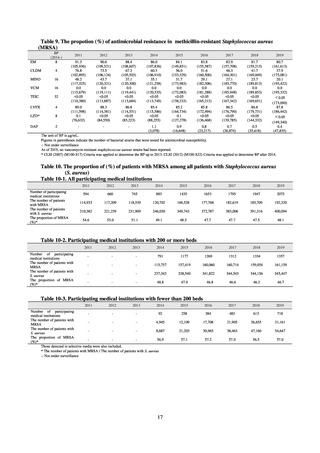
Figure 5. System for antimicrobial resistance monitoring in healthy dogs and cats (from FY2018)
Food Safety and Consumer Affairs Bureau, MAFF
Request for
cooperation
Public results
Contract the
project
Japan Veterinary
Medical Association
Request for
cooperation
Veterinary hospital
Report
National Veterinary Assay
Laboratory
Bacterial strains
and data
- Stoch bacterial strains
- Conduct molecular
epdemiological surveys, etc.
Contract laboratories
- Isolate and identify bacteria
(E. coli, Enterococcus spp.)
- Antimicrobial susceptibility
tests
Rectal swab
*In principle, 1 sample per
animal species per hospital
Veterinary hospital
Veterinary hospital
Quality control
Veterinary hospital
Veterinary hospital
3) Monitoring on the sales volumes of antimicrobials
An annual monitoring is conducted on the volumes of sales of veterinary antimicrobials, based on the reported quantities
of veterinary drugs handled by marketing authorization holders, pursuant to Article 71-2 of the Veterinary Drug Control
Regulations (MAFF Ordinance No. 107 of 2004) (Figure 6). Starting 2001, the monitoring has included the volume of
sales by active pharmaceutical ingredient, and the estimated percentage of sales by animal species, in addition to the
volumes of sales by antimicrobial class and route of administration. The data are aggregated and published on the website
of the National Veterinary Assay Laboratory as “Annual Report of Sales Amount and Sales Volume of Veterinary drugs,
Quasi-drugs and Medical Devices.” Under the OIE Terrestrial Animal Health Code’s section on antimicrobial usage
(Chapter 6.8), [4] these data are submitted to the OIE for the activity to understand and compare usage in each country of
the world.
94
関連画像
ページ内で利用されている画像ファイルです。