よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (69 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
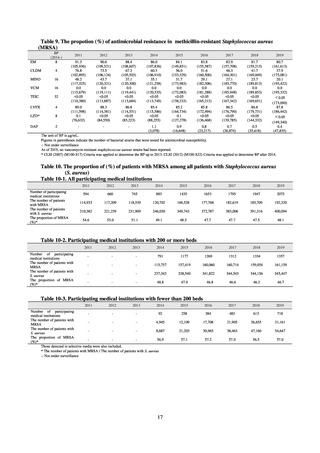
3) Companion animals
The estimated volumes of veterinary antimicrobials sold for companion animals (dogs and cats) in terms of
active ingredients are summarized in Table 79. In the period from 2013 to 2018, the estimated volume of sales
ranged between 7.79 t and 9.67 t, accounting for between 0.9% and 1.2 % of the total volume of veterinary
antimicrobial sales. Use of human antimicrobials in companion animals was not originally monitored under
JVARM and is therefore excluded from the values in the table for 2015 and earlier. Accordingly, with the full
cooperation of the Japan Animal Drugs & Instruments Dealers Association and Federation of Japan
Pharmaceutical Wholesalers Association, the Ministry of Agriculture, Forestry and Fisheries began monitoring
the actual usage of human antimicrobials in 2016 based on the amount sold. The results of its surveillance revealed
that the volume of human antimicrobials sold for use in companion animals is about the same as the volume of
veterinary antimicrobials sold for that purpose. Including those for human use, the most commonly sold
antimicrobials were first-generation cephalosporins and penicillins.
Table 79. The estimated volumes of sales of veterinary and human antimicrobials for companion animals
(cats and dogs) in terms of active ingredients (unit: metric tons)
2013
2014
2015
2017
2018
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
2016
Human
antimicrobi
als
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
Penicillins
2.36
2.13
2.08
1.57
1.93
1.68
1.66
Cephalosporins (total)
2.45
2.44
2.67
3.12
3.23
3.21
3.16
1st generation cephalosporins
(2.26)
(2.23)
(2.46)
(2.89)
(2.99)
(2.93)
2nd generation cephalosporins
(0.00)
(0.00)
(0.00)
(0.00)
(0.00)
(0.00)
3rd generation cephalosporins
(0.20)
(0.20)
(0.21)
(0.23)
(0.11)
(0.22)
(0.22)
Aminoglycosides
2.07
1.97
1.40
0.41
0.02
0.39
0.91
Macrolides
0.00
0.00
0.00
0.00
0.17
0.00
0.02
Lincosamides
0.09
0.09
0.11
0.13
0.10
0.13
0.14
Tetracyclines
0.00
0.00
0.00
0.00
0.28
0.00
1.27
Peptides
0.01
0.01
0.01
0.01
0.00
0.01
0.01
Other antibioitics**
0.00
0.00
0.00
0.00
0.22
0.00
0.00
Sulfonamides
0.60
0.55
0.56
0.53
0.19
0.57
0.53
Quinolones
0.00
0.00
0.00
0.00
0.00
0.00
0.00
Fluoroquinolones
0.90
0.90
0.94
0.89
0.11
0.90
0.84
Amphenicols
0.00
0.00
0.00
0.00
0.12
0.01
0.01
Furan and derivatives
0.00
0.00
0.00
0.00
0.00
0.00
0.00
Other synthetic antibacterials***
0.02
0.01
0.01
0.01
0.08
0.01
0.01
Antifungal antibiotics
1.18
1.03
1.08
1.12
0.00
1.07
1.06
Total
9.67
9.13
8.86
7.79
6.48
7.97
9.62
(3.12)
* The figures in parentheses are included in the Cephalosporins (total).
** Includes fosfomycin and rifamycin.
*** Includes trimethoprim, penems, carbapenems, isoniazid, and ethambutol.
(3) Antimicrobial feed additives
Source: Food and Agricultural Materials Inspection Center (FAMIC) and Japan Scientific Feeds Association
The volumes of distribution of antimicrobial feed additives, based on surveys by the Food and Agricultural
Materials Inspection Center and by the Japan Scientific Feeds Association, are indicated in Table 80. While the
volume of such additives distributed showed a moderate decline in the period 2013 to 2018, ranging between 235.1
t and 216.7 t, comparisons among the different types of antimicrobials showed an upward trend in the distribution
of polyethers (not used in humans), which account for the majority. The designation of the polypeptide colistin as
a feed additive was revoked in July 2018, followed by the macrolide tylosin in May 2019 and two tetracyclines in
December 2019. Distribution of these antimicrobials ceased from the time their designation was revoked.
68
The estimated volumes of veterinary antimicrobials sold for companion animals (dogs and cats) in terms of
active ingredients are summarized in Table 79. In the period from 2013 to 2018, the estimated volume of sales
ranged between 7.79 t and 9.67 t, accounting for between 0.9% and 1.2 % of the total volume of veterinary
antimicrobial sales. Use of human antimicrobials in companion animals was not originally monitored under
JVARM and is therefore excluded from the values in the table for 2015 and earlier. Accordingly, with the full
cooperation of the Japan Animal Drugs & Instruments Dealers Association and Federation of Japan
Pharmaceutical Wholesalers Association, the Ministry of Agriculture, Forestry and Fisheries began monitoring
the actual usage of human antimicrobials in 2016 based on the amount sold. The results of its surveillance revealed
that the volume of human antimicrobials sold for use in companion animals is about the same as the volume of
veterinary antimicrobials sold for that purpose. Including those for human use, the most commonly sold
antimicrobials were first-generation cephalosporins and penicillins.
Table 79. The estimated volumes of sales of veterinary and human antimicrobials for companion animals
(cats and dogs) in terms of active ingredients (unit: metric tons)
2013
2014
2015
2017
2018
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
2016
Human
antimicrobi
als
Veterinary
antimicrobi
als
Veterinary
antimicrobi
als
Penicillins
2.36
2.13
2.08
1.57
1.93
1.68
1.66
Cephalosporins (total)
2.45
2.44
2.67
3.12
3.23
3.21
3.16
1st generation cephalosporins
(2.26)
(2.23)
(2.46)
(2.89)
(2.99)
(2.93)
2nd generation cephalosporins
(0.00)
(0.00)
(0.00)
(0.00)
(0.00)
(0.00)
3rd generation cephalosporins
(0.20)
(0.20)
(0.21)
(0.23)
(0.11)
(0.22)
(0.22)
Aminoglycosides
2.07
1.97
1.40
0.41
0.02
0.39
0.91
Macrolides
0.00
0.00
0.00
0.00
0.17
0.00
0.02
Lincosamides
0.09
0.09
0.11
0.13
0.10
0.13
0.14
Tetracyclines
0.00
0.00
0.00
0.00
0.28
0.00
1.27
Peptides
0.01
0.01
0.01
0.01
0.00
0.01
0.01
Other antibioitics**
0.00
0.00
0.00
0.00
0.22
0.00
0.00
Sulfonamides
0.60
0.55
0.56
0.53
0.19
0.57
0.53
Quinolones
0.00
0.00
0.00
0.00
0.00
0.00
0.00
Fluoroquinolones
0.90
0.90
0.94
0.89
0.11
0.90
0.84
Amphenicols
0.00
0.00
0.00
0.00
0.12
0.01
0.01
Furan and derivatives
0.00
0.00
0.00
0.00
0.00
0.00
0.00
Other synthetic antibacterials***
0.02
0.01
0.01
0.01
0.08
0.01
0.01
Antifungal antibiotics
1.18
1.03
1.08
1.12
0.00
1.07
1.06
Total
9.67
9.13
8.86
7.79
6.48
7.97
9.62
(3.12)
* The figures in parentheses are included in the Cephalosporins (total).
** Includes fosfomycin and rifamycin.
*** Includes trimethoprim, penems, carbapenems, isoniazid, and ethambutol.
(3) Antimicrobial feed additives
Source: Food and Agricultural Materials Inspection Center (FAMIC) and Japan Scientific Feeds Association
The volumes of distribution of antimicrobial feed additives, based on surveys by the Food and Agricultural
Materials Inspection Center and by the Japan Scientific Feeds Association, are indicated in Table 80. While the
volume of such additives distributed showed a moderate decline in the period 2013 to 2018, ranging between 235.1
t and 216.7 t, comparisons among the different types of antimicrobials showed an upward trend in the distribution
of polyethers (not used in humans), which account for the majority. The designation of the polypeptide colistin as
a feed additive was revoked in July 2018, followed by the macrolide tylosin in May 2019 and two tetracyclines in
December 2019. Distribution of these antimicrobials ceased from the time their designation was revoked.
68