よむ、つかう、まなぶ。
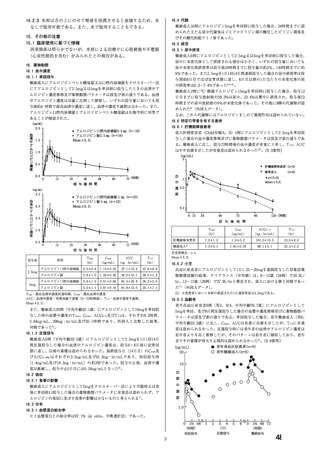
資料1-2 アムロジピンベシル酸塩 調査結果報告書及び添付文書 (20 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe
hypertension can provide substantial benefit. Relative risk reduction from blood pressure
reduction is similar across populations with varying absolute risk, so the absolute benefit is
greater in patients who are at higher risk independent of their hypertension (for example, patients
with diabetes or hyperlipidemia), and such patients would be expected to benefit from more
aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black
patients, and many antihypertensive drugs have additional approved indications and effects (e.g.,
on angina, heart failure, or diabetic kidney disease). These considerations may guide selection
of therapy.
KATERZIA may be used alone or in combination with other antihypertensive agents.
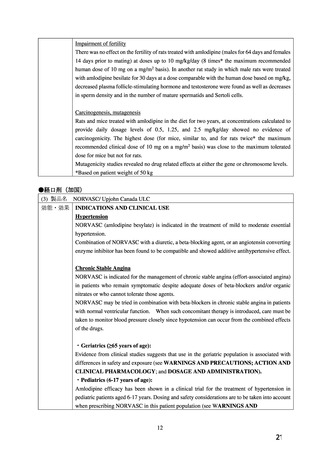
1.2 Coronary Artery Disease (CAD)
Chronic Stable Angina
KATERZIA is indicated for the symptomatic treatment of chronic stable angina. KATERZIA
may be used alone or in combination with other antianginal agents.
Vasospastic Angina (Prinzmetal’s or Variant Angina)
KATERZIA is indicated for the treatment of confirmed or suspected vasospastic angina.
KATERZIA may be used as monotherapy or in combination with other antianginal agents.
Angiographically Documented CAD
In patients with recently documented CAD by angiography and without heart failure or an
ejection fraction <40%, KATERZIA is indicated to reduce the risk of hospitalization for angina
and to reduce the risk of a coronary revascularization procedure.
妊婦への
8 USE IN SPECIFIC POPULATIONS
投与
8.1 Pregnancy
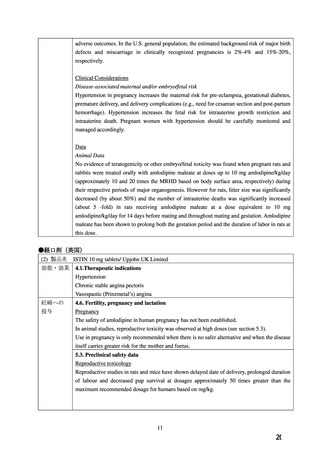
Risk Summary
The limited available data based on post-marketing reports with amlodipine use in pregnant
women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage.
There are risks to the mother and fetus associated with poorly controlled hypertension in
pregnancy (see Clinical Considerations). In animal reproduction studies, there was no evidence
of adverse developmental effects when pregnant rats and rabbits were treated orally with
amlodipine maleate during organogenesis at doses approximately 10 and 20-times the maximum
recommended human dose (MRHD), respectively. However for rats, litter size was significantly
decreased (by about 50%) and the number of intrauterine deaths was significantly increased
(about 5-fold). Amlodipine has been shown to prolong both the gestation period and the duration
of labor in rats at this dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated
population is unknown. All pregnancies have a background risk of birth defect, loss or other
10
19
hypertension can provide substantial benefit. Relative risk reduction from blood pressure
reduction is similar across populations with varying absolute risk, so the absolute benefit is
greater in patients who are at higher risk independent of their hypertension (for example, patients
with diabetes or hyperlipidemia), and such patients would be expected to benefit from more
aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black
patients, and many antihypertensive drugs have additional approved indications and effects (e.g.,
on angina, heart failure, or diabetic kidney disease). These considerations may guide selection
of therapy.
KATERZIA may be used alone or in combination with other antihypertensive agents.
1.2 Coronary Artery Disease (CAD)
Chronic Stable Angina
KATERZIA is indicated for the symptomatic treatment of chronic stable angina. KATERZIA
may be used alone or in combination with other antianginal agents.
Vasospastic Angina (Prinzmetal’s or Variant Angina)
KATERZIA is indicated for the treatment of confirmed or suspected vasospastic angina.
KATERZIA may be used as monotherapy or in combination with other antianginal agents.
Angiographically Documented CAD
In patients with recently documented CAD by angiography and without heart failure or an
ejection fraction <40%, KATERZIA is indicated to reduce the risk of hospitalization for angina
and to reduce the risk of a coronary revascularization procedure.
妊婦への
8 USE IN SPECIFIC POPULATIONS
投与
8.1 Pregnancy
Risk Summary
The limited available data based on post-marketing reports with amlodipine use in pregnant
women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage.
There are risks to the mother and fetus associated with poorly controlled hypertension in
pregnancy (see Clinical Considerations). In animal reproduction studies, there was no evidence
of adverse developmental effects when pregnant rats and rabbits were treated orally with
amlodipine maleate during organogenesis at doses approximately 10 and 20-times the maximum
recommended human dose (MRHD), respectively. However for rats, litter size was significantly
decreased (by about 50%) and the number of intrauterine deaths was significantly increased
(about 5-fold). Amlodipine has been shown to prolong both the gestation period and the duration
of labor in rats at this dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated
population is unknown. All pregnancies have a background risk of birth defect, loss or other
10
19