よむ、つかう、まなぶ。
資料1-2 アムロジピンベシル酸塩 調査結果報告書及び添付文書 (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
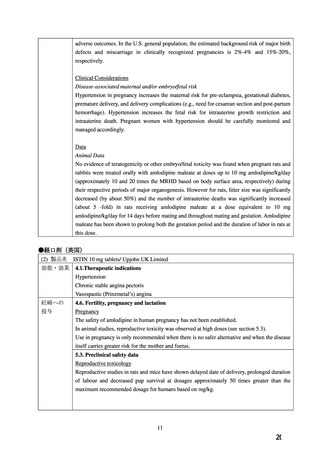
PRECAUTIONS; ACTION AND CLINICAL PHARMACOLOGY; and DOSAGE AND
ADMINISTRATION).
The use of NORVASC in children less than 6 years of age is not recommended (see
WARNINGS AND PRECAUTIONS - Special Populations).
妊婦への
WARNINGS AND PRECAUTIONS
投与
Special Populations
Pregnant Women: There is no clinical experience with NORVASC in pregnant women.
NORVASC should be used during pregnancy only if the potential benefit outweighs the
potential risk to the mother and fetus.
Although amlodipine was not teratogenic in the rat and rabbit some dihydropyridine compounds
have been found to be teratogenic in animals. In rats, amlodipine has been shown to prolong
both the gestation period and the duration of labor. There was no effect on the fertility of rats
treated with amlodipine.
●経口剤(豪州)
(4) 製品名 NORVASC / Aspen Pharmacare Australia Pty Ltd
効能・効果
4.1 Therapeutic indications
1. Hypertension
NORVASC is indicated for the first line treatment of hypertension and can be used as the sole
agent to control blood pressure in the majority of patients. Patients not adequately controlled on
a single antihypertensive agent may benefit from the addition of NORVASC, which has been
used in combination with a thiazide diuretic, beta adrenoceptor blocking agent or an angiotensinconverting enzyme inhibitor.
2. Angina
NORVASC is indicated for the first line treatment of chronic stable angina. NORVASC may
be used alone, as monotherapy or in combination with other antianginal drugs.
妊婦への
4.6 Fertility, pregnancy and lactation
投与
Use in pregnancy - Category C.
Calcium channel blockers carry the potential to produce fetal hypoxia associated with maternal
hypotension. Accordingly they should not be used in pregnant women unless the potential
benefit outweighs the risk to the fetus.
The safety of NORVASC in human pregnancy or lactation has not been established.
Amlodipine (10 mg/kg as besilate salt, 7 mg/kg base), administered orally to rats at or near
parturition induced a prolongation of gestation time, an increase in the number of stillbirths and
a decreased postnatal survival.
13
22
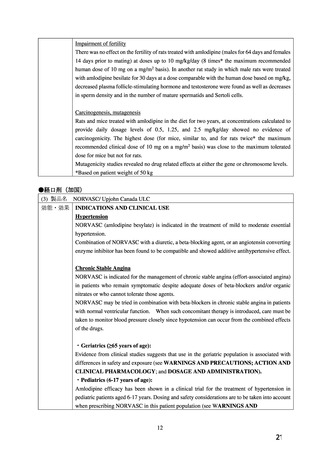
ADMINISTRATION).
The use of NORVASC in children less than 6 years of age is not recommended (see
WARNINGS AND PRECAUTIONS - Special Populations).
妊婦への
WARNINGS AND PRECAUTIONS
投与
Special Populations
Pregnant Women: There is no clinical experience with NORVASC in pregnant women.
NORVASC should be used during pregnancy only if the potential benefit outweighs the
potential risk to the mother and fetus.
Although amlodipine was not teratogenic in the rat and rabbit some dihydropyridine compounds
have been found to be teratogenic in animals. In rats, amlodipine has been shown to prolong
both the gestation period and the duration of labor. There was no effect on the fertility of rats
treated with amlodipine.
●経口剤(豪州)
(4) 製品名 NORVASC / Aspen Pharmacare Australia Pty Ltd
効能・効果
4.1 Therapeutic indications
1. Hypertension
NORVASC is indicated for the first line treatment of hypertension and can be used as the sole
agent to control blood pressure in the majority of patients. Patients not adequately controlled on
a single antihypertensive agent may benefit from the addition of NORVASC, which has been
used in combination with a thiazide diuretic, beta adrenoceptor blocking agent or an angiotensinconverting enzyme inhibitor.
2. Angina
NORVASC is indicated for the first line treatment of chronic stable angina. NORVASC may
be used alone, as monotherapy or in combination with other antianginal drugs.
妊婦への
4.6 Fertility, pregnancy and lactation
投与
Use in pregnancy - Category C.
Calcium channel blockers carry the potential to produce fetal hypoxia associated with maternal
hypotension. Accordingly they should not be used in pregnant women unless the potential
benefit outweighs the risk to the fetus.
The safety of NORVASC in human pregnancy or lactation has not been established.
Amlodipine (10 mg/kg as besilate salt, 7 mg/kg base), administered orally to rats at or near
parturition induced a prolongation of gestation time, an increase in the number of stillbirths and
a decreased postnatal survival.
13
22