よむ、つかう、まなぶ。
資料1-2 アムロジピンベシル酸塩 調査結果報告書及び添付文書 (22 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
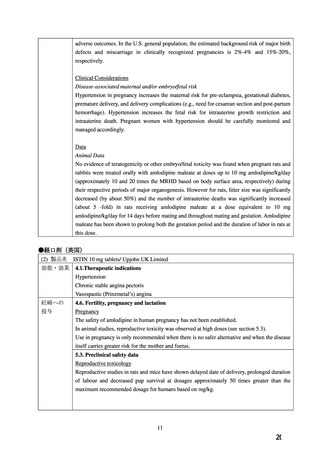
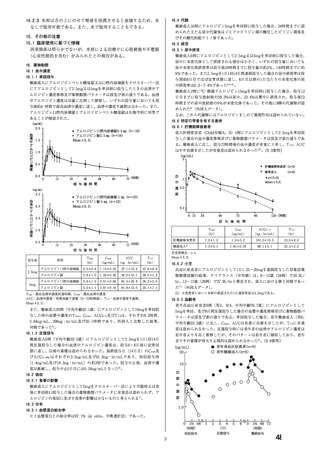
Impairment of fertility
There was no effect on the fertility of rats treated with amlodipine (males for 64 days and females
14 days prior to mating) at doses up to 10 mg/kg/day (8 times* the maximum recommended
human dose of 10 mg on a mg/m2 basis). In another rat study in which male rats were treated
with amlodipine besilate for 30 days at a dose comparable with the human dose based on mg/kg,
decreased plasma follicle-stimulating hormone and testosterone were found as well as decreases
in sperm density and in the number of mature spermatids and Sertoli cells.
Carcinogenesis, mutagenesis
Rats and mice treated with amlodipine in the diet for two years, at concentrations calculated to
provide daily dosage levels of 0.5, 1.25, and 2.5 mg/kg/day showed no evidence of
carcinogenicity. The highest dose (for mice, similar to, and for rats twice* the maximum
recommended clinical dose of 10 mg on a mg/m2 basis) was close to the maximum tolerated
dose for mice but not for rats.
Mutagenicity studies revealed no drug related effects at either the gene or chromosome levels.
*Based on patient weight of 50 kg
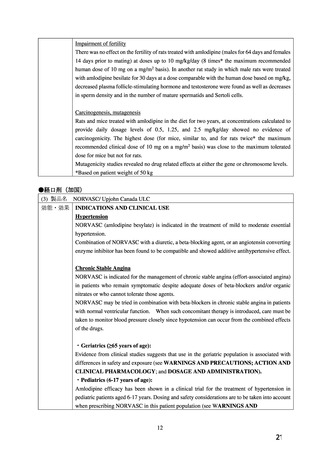
●経口剤(加国)
(3) 製品名 NORVASC/ Upjohn Canada ULC
効能・効果
INDICATIONS AND CLINICAL USE
Hypertension
NORVASC (amlodipine besylate) is indicated in the treatment of mild to moderate essential
hypertension.
Combination of NORVASC with a diuretic, a beta-blocking agent, or an angiotensin converting
enzyme inhibitor has been found to be compatible and showed additive antihypertensive effect.
Chronic Stable Angina
NORVASC is indicated for the management of chronic stable angina (effort-associated angina)
in patients who remain symptomatic despite adequate doses of beta-blockers and/or organic
nitrates or who cannot tolerate those agents.
NORVASC may be tried in combination with beta-blockers in chronic stable angina in patients
with normal ventricular function. When such concomitant therapy is introduced, care must be
taken to monitor blood pressure closely since hypotension can occur from the combined effects
of the drugs.
・Geriatrics (≥65 years of age):
Evidence from clinical studies suggests that use in the geriatric population is associated with
differences in safety and exposure (see WARNINGS AND PRECAUTIONS; ACTION AND
CLINICAL PHARMACOLOGY; and DOSAGE AND ADMINISTRATION).
・Pediatrics (6-17 years of age):
Amlodipine efficacy has been shown in a clinical trial for the treatment of hypertension in
pediatric patients aged 6-17 years. Dosing and safety considerations are to be taken into account
when prescribing NORVASC in this patient population (see WARNINGS AND
12
21
There was no effect on the fertility of rats treated with amlodipine (males for 64 days and females
14 days prior to mating) at doses up to 10 mg/kg/day (8 times* the maximum recommended
human dose of 10 mg on a mg/m2 basis). In another rat study in which male rats were treated
with amlodipine besilate for 30 days at a dose comparable with the human dose based on mg/kg,
decreased plasma follicle-stimulating hormone and testosterone were found as well as decreases
in sperm density and in the number of mature spermatids and Sertoli cells.
Carcinogenesis, mutagenesis
Rats and mice treated with amlodipine in the diet for two years, at concentrations calculated to
provide daily dosage levels of 0.5, 1.25, and 2.5 mg/kg/day showed no evidence of
carcinogenicity. The highest dose (for mice, similar to, and for rats twice* the maximum
recommended clinical dose of 10 mg on a mg/m2 basis) was close to the maximum tolerated
dose for mice but not for rats.
Mutagenicity studies revealed no drug related effects at either the gene or chromosome levels.
*Based on patient weight of 50 kg
●経口剤(加国)
(3) 製品名 NORVASC/ Upjohn Canada ULC
効能・効果
INDICATIONS AND CLINICAL USE
Hypertension
NORVASC (amlodipine besylate) is indicated in the treatment of mild to moderate essential
hypertension.
Combination of NORVASC with a diuretic, a beta-blocking agent, or an angiotensin converting
enzyme inhibitor has been found to be compatible and showed additive antihypertensive effect.
Chronic Stable Angina
NORVASC is indicated for the management of chronic stable angina (effort-associated angina)
in patients who remain symptomatic despite adequate doses of beta-blockers and/or organic
nitrates or who cannot tolerate those agents.
NORVASC may be tried in combination with beta-blockers in chronic stable angina in patients
with normal ventricular function. When such concomitant therapy is introduced, care must be
taken to monitor blood pressure closely since hypotension can occur from the combined effects
of the drugs.
・Geriatrics (≥65 years of age):
Evidence from clinical studies suggests that use in the geriatric population is associated with
differences in safety and exposure (see WARNINGS AND PRECAUTIONS; ACTION AND
CLINICAL PHARMACOLOGY; and DOSAGE AND ADMINISTRATION).
・Pediatrics (6-17 years of age):
Amlodipine efficacy has been shown in a clinical trial for the treatment of hypertension in
pediatric patients aged 6-17 years. Dosing and safety considerations are to be taken into account
when prescribing NORVASC in this patient population (see WARNINGS AND
12
21