よむ、つかう、まなぶ。
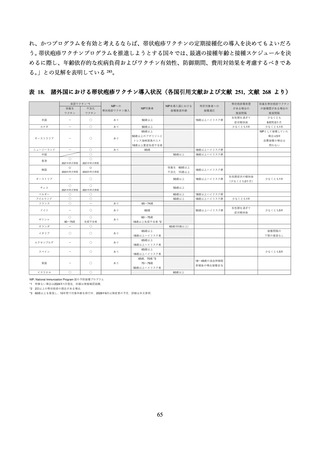
03 資料1-1 帯状疱疹ワクチンファクトシート (78 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_40826.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第26回 6/20)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
193.
Lee E, Chun JY, Song KH, et al. Optimal Timing of Zoster Vaccination After Shingles: A Prospective Study of the
Immunogenicity and Safety of Live Zoster Vaccine. Infect Chemother 2018; 50(4): 311-8.
194.
Ohfuji S, Ito K, Inoue M, et al. Safety of live attenuated varicella-zoster vaccine in patients with underlying
illnesses compared with healthy adults: a prospective cohort study. BMC Infect Dis 2019; 19(1): 95.
195.
Naidus E, Damon L, Schwartz BS, Breed C, Liu C. Experience with use of Zostavax(®) in patients with
hematologic malignancy and hematopoietic cell transplant recipients. Am J Hematol 2012; 87(1): 123-5.
196.
Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster
infection among older patients with selected immune-mediated diseases. Jama 2012; 308(1): 43-9.
197.
Cheetham TC, Marcy SM, Tseng HF, et al. Risk of Herpes Zoster and Disseminated Varicella Zoster in Patients
Taking Immunosuppressant Drugs at the Time of Zoster Vaccination. Mayo Clin Proc 2015; 90(7): 865-73.
198.
Russell AF, Parrino J, Fisher CL, Jr., et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on
chronic/maintenance corticosteroids. Vaccine 2015; 33(27): 3129-34.
199.
Ortiz-Brizuela E, Leal-Vega F, Cuellar-Rodríguez J, Bobadilla-Del-Valle M, Ponce-de-León A. Vaccine-derived
varicella zoster infection in a kidney transplant recipient after zoster vaccine live administration. Vaccine 2019;
37(27): 3576-9.
200.
Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Bühler S. Safety of live vaccinations on immunosuppressive
therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow
transplantation - A systematic review of randomized trials, observational studies and case reports. Vaccine 2017;
35(9): 1216-26.
201.
Chun JY, Kim K, Lee MK, et al. Immunogenicity and safety of a live herpes zoster vaccine in hematopoietic stem
cell transplant recipients. BMC Infect Dis 2021; 21(1): 117.
202.
Wang L, Verschuuren EAM, Paap D, et al. Prophylactic vaccination with a live-attenuated herpes zoster vaccine in
lung transplant candidates. J Heart Lung Transplant 2020; 39(12): 1445-54.
203.
Chong PP, Avery RK. A Comprehensive Review of Immunization Practices in Solid Organ Transplant and
Hematopoietic Stem Cell Transplant Recipients. Clin Ther 2017; 39(8): 1581-98.
204.
Tran CT, Ducancelle A, Masson C, Lunel-Fabiani F. Herpes zoster: Risk and prevention during
immunomodulating therapy. Joint Bone Spine 2017; 84(1): 21-7.
205.
Levin MJ, Bresnitz E, Popmihajlov Z, et al. Studies with herpes zoster vaccines in immune compromised patients.
Expert Rev Vaccines 2017; 16(12): 1217-30.
206.
Khan N, Shah Y, Trivedi C, Lewis JD. Safety of herpes zoster vaccination among inflammatory bowel disease
patients being treated with anti-TNF medications. Aliment Pharmacol Ther 2017; 46(7): 668-72.
207.
Guillo L, Rabaud C, Choy EH, et al. Herpes Zoster and Vaccination Strategies in Inflammatory Bowel Diseases: A
Practical Guide. Clin Gastroenterol Hepatol 2022; 20(3): 481-90.
208.
Wasan SK, Zullow S, Berg A, Cheifetz AS, Ganley-Leal L, Farraye FA. Herpes Zoster Vaccine Response in
Inflammatory Bowel Disease Patients on Low-dose Immunosuppression. Inflamm Bowel Dis 2016; 22(6): 1391-6.
209.
Benchimol EI, Tse F, Carroll MW, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for
Immunizations in Patients With Inflammatory Bowel Disease (IBD)-Part 1: Live Vaccines. J Can Assoc
Gastroenterol 2021; 4(4): e59-e71.
77
Lee E, Chun JY, Song KH, et al. Optimal Timing of Zoster Vaccination After Shingles: A Prospective Study of the
Immunogenicity and Safety of Live Zoster Vaccine. Infect Chemother 2018; 50(4): 311-8.
194.
Ohfuji S, Ito K, Inoue M, et al. Safety of live attenuated varicella-zoster vaccine in patients with underlying
illnesses compared with healthy adults: a prospective cohort study. BMC Infect Dis 2019; 19(1): 95.
195.
Naidus E, Damon L, Schwartz BS, Breed C, Liu C. Experience with use of Zostavax(®) in patients with
hematologic malignancy and hematopoietic cell transplant recipients. Am J Hematol 2012; 87(1): 123-5.
196.
Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster
infection among older patients with selected immune-mediated diseases. Jama 2012; 308(1): 43-9.
197.
Cheetham TC, Marcy SM, Tseng HF, et al. Risk of Herpes Zoster and Disseminated Varicella Zoster in Patients
Taking Immunosuppressant Drugs at the Time of Zoster Vaccination. Mayo Clin Proc 2015; 90(7): 865-73.
198.
Russell AF, Parrino J, Fisher CL, Jr., et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on
chronic/maintenance corticosteroids. Vaccine 2015; 33(27): 3129-34.
199.
Ortiz-Brizuela E, Leal-Vega F, Cuellar-Rodríguez J, Bobadilla-Del-Valle M, Ponce-de-León A. Vaccine-derived
varicella zoster infection in a kidney transplant recipient after zoster vaccine live administration. Vaccine 2019;
37(27): 3576-9.
200.
Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Bühler S. Safety of live vaccinations on immunosuppressive
therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow
transplantation - A systematic review of randomized trials, observational studies and case reports. Vaccine 2017;
35(9): 1216-26.
201.
Chun JY, Kim K, Lee MK, et al. Immunogenicity and safety of a live herpes zoster vaccine in hematopoietic stem
cell transplant recipients. BMC Infect Dis 2021; 21(1): 117.
202.
Wang L, Verschuuren EAM, Paap D, et al. Prophylactic vaccination with a live-attenuated herpes zoster vaccine in
lung transplant candidates. J Heart Lung Transplant 2020; 39(12): 1445-54.
203.
Chong PP, Avery RK. A Comprehensive Review of Immunization Practices in Solid Organ Transplant and
Hematopoietic Stem Cell Transplant Recipients. Clin Ther 2017; 39(8): 1581-98.
204.
Tran CT, Ducancelle A, Masson C, Lunel-Fabiani F. Herpes zoster: Risk and prevention during
immunomodulating therapy. Joint Bone Spine 2017; 84(1): 21-7.
205.
Levin MJ, Bresnitz E, Popmihajlov Z, et al. Studies with herpes zoster vaccines in immune compromised patients.
Expert Rev Vaccines 2017; 16(12): 1217-30.
206.
Khan N, Shah Y, Trivedi C, Lewis JD. Safety of herpes zoster vaccination among inflammatory bowel disease
patients being treated with anti-TNF medications. Aliment Pharmacol Ther 2017; 46(7): 668-72.
207.
Guillo L, Rabaud C, Choy EH, et al. Herpes Zoster and Vaccination Strategies in Inflammatory Bowel Diseases: A
Practical Guide. Clin Gastroenterol Hepatol 2022; 20(3): 481-90.
208.
Wasan SK, Zullow S, Berg A, Cheifetz AS, Ganley-Leal L, Farraye FA. Herpes Zoster Vaccine Response in
Inflammatory Bowel Disease Patients on Low-dose Immunosuppression. Inflamm Bowel Dis 2016; 22(6): 1391-6.
209.
Benchimol EI, Tse F, Carroll MW, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for
Immunizations in Patients With Inflammatory Bowel Disease (IBD)-Part 1: Live Vaccines. J Can Assoc
Gastroenterol 2021; 4(4): e59-e71.
77