よむ、つかう、まなぶ。
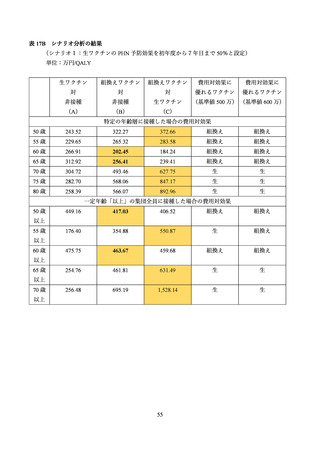
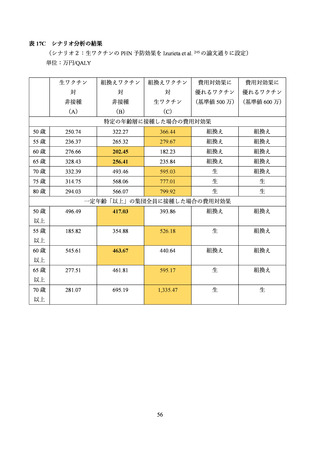
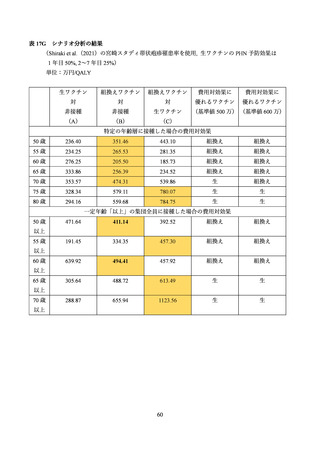
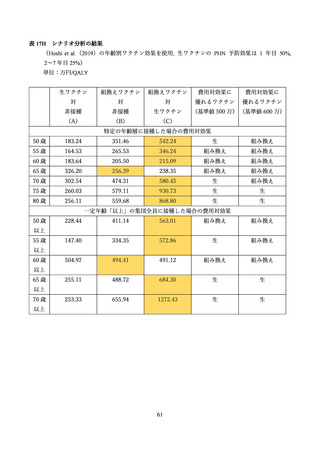
03 資料1-1 帯状疱疹ワクチンファクトシート (80 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_40826.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第26回 6/20)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Using Electronic Health Record Data. Am J Epidemiol 2023; 192(2): 205-16.
226.
Godeaux O, Kovac M, Shu D, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate
vaccine in adults >/= 50 years of age with a prior history of herpes zoster: A phase III, non-randomized, open-label
clinical trial. Hum Vaccin Immunother 2017; 13(5): 1051-8.
227.
Grupping K, Campora L, Douha M, et al. Immunogenicity and Safety of the HZ/su Adjuvanted Herpes Zoster
Subunit Vaccine in Adults Previously Vaccinated With a Live Attenuated Herpes Zoster Vaccine. J Infect Dis 2017;
216(11): 1343-51.
228.
Bastidas A, de la Serna J, El Idrissi M, et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster
After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019; 322(2): 123-33.
229.
Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in
adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet
Infect Dis 2019; 19(9): 988-1000.
230.
Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted
recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: A randomized
trial. Cancer 2019; 125(8): 1301-12.
231.
Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and Safety of the Adjuvanted
Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3,
Randomized Clinical Trial. Clin Infect Dis 2020; 70(2): 181-90.
232.
Dagnew AF, Rausch D, Herve C, et al. Efficacy and serious adverse events profile of the adjuvanted recombinant
zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two
parallel randomized trials. Rheumatology (Oxford) 2021; 60(3): 1226-33.
233.
Stevens E, Weinblatt ME, Massarotti E, Griffin F, Emani S, Desai S. Safety of the Zoster Vaccine Recombinant
Adjuvanted in Rheumatoid Arthritis and Other Systemic Rheumatic Disease Patients: A Single Center's Experience
With 400 Patients. ACR Open Rheumatol 2020; 2(6): 357-61.
234.
伊藤聡, 船村啓, 阿部麻美, et al. Safety and influence of the recombinant zoster vaccine on disease activity in
Japanese patients with rheumatic diseases. 臨床リウマチ 2023; 35(2): 64-76.
235.
Källmark H, Bergström T, Nagel J, et al. Serologic immunogenicity and safety of Herpes Zoster subunit vaccine in
patients with Rheumatoid Arthritis receiving Janus Kinase inhibitors. Rheumatology (Oxford) 2023.
236.
Venerito V, Stefanizzi P, Cantarini L, et al. Immunogenicity and Safety of Adjuvanted Recombinant Zoster
Vaccine in Rheumatoid Arthritis Patients on Anti-Cellular Biologic Agents or JAK Inhibitors: A Prospective
Observational Study. Int J Mol Sci 2023; 24(8).
237.
Esteban-Vazquez A, Steiner M, Castaneda E, et al. The Real-World Study of Immunogenicity and Safety of the
Adjuvant Recombinant Vaccine against Varicella Zoster Virus in Patients with Immune-Mediated Inflammatory
Diseases Treated with Janus Kinase Inhibitors. Vaccines (Basel) 2023; 11(10).
238.
Khan N, Trivedi C, Aberra F, Pernes T, Yang YX. Safety of Recombinant Zoster Vaccine in Patients with
Inflammatory Bowel Disease. J Crohns Colitis 2022; 16(9): 1505-7.
239.
Satyam VR, Li PH, Reich J, et al. Safety of Recombinant Zoster Vaccine in Patients with Inflammatory Bowel
Disease. Dig Dis Sci 2020; 65(10): 2986-91.
79
226.
Godeaux O, Kovac M, Shu D, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate
vaccine in adults >/= 50 years of age with a prior history of herpes zoster: A phase III, non-randomized, open-label
clinical trial. Hum Vaccin Immunother 2017; 13(5): 1051-8.
227.
Grupping K, Campora L, Douha M, et al. Immunogenicity and Safety of the HZ/su Adjuvanted Herpes Zoster
Subunit Vaccine in Adults Previously Vaccinated With a Live Attenuated Herpes Zoster Vaccine. J Infect Dis 2017;
216(11): 1343-51.
228.
Bastidas A, de la Serna J, El Idrissi M, et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster
After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019; 322(2): 123-33.
229.
Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in
adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet
Infect Dis 2019; 19(9): 988-1000.
230.
Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted
recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: A randomized
trial. Cancer 2019; 125(8): 1301-12.
231.
Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and Safety of the Adjuvanted
Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3,
Randomized Clinical Trial. Clin Infect Dis 2020; 70(2): 181-90.
232.
Dagnew AF, Rausch D, Herve C, et al. Efficacy and serious adverse events profile of the adjuvanted recombinant
zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two
parallel randomized trials. Rheumatology (Oxford) 2021; 60(3): 1226-33.
233.
Stevens E, Weinblatt ME, Massarotti E, Griffin F, Emani S, Desai S. Safety of the Zoster Vaccine Recombinant
Adjuvanted in Rheumatoid Arthritis and Other Systemic Rheumatic Disease Patients: A Single Center's Experience
With 400 Patients. ACR Open Rheumatol 2020; 2(6): 357-61.
234.
伊藤聡, 船村啓, 阿部麻美, et al. Safety and influence of the recombinant zoster vaccine on disease activity in
Japanese patients with rheumatic diseases. 臨床リウマチ 2023; 35(2): 64-76.
235.
Källmark H, Bergström T, Nagel J, et al. Serologic immunogenicity and safety of Herpes Zoster subunit vaccine in
patients with Rheumatoid Arthritis receiving Janus Kinase inhibitors. Rheumatology (Oxford) 2023.
236.
Venerito V, Stefanizzi P, Cantarini L, et al. Immunogenicity and Safety of Adjuvanted Recombinant Zoster
Vaccine in Rheumatoid Arthritis Patients on Anti-Cellular Biologic Agents or JAK Inhibitors: A Prospective
Observational Study. Int J Mol Sci 2023; 24(8).
237.
Esteban-Vazquez A, Steiner M, Castaneda E, et al. The Real-World Study of Immunogenicity and Safety of the
Adjuvant Recombinant Vaccine against Varicella Zoster Virus in Patients with Immune-Mediated Inflammatory
Diseases Treated with Janus Kinase Inhibitors. Vaccines (Basel) 2023; 11(10).
238.
Khan N, Trivedi C, Aberra F, Pernes T, Yang YX. Safety of Recombinant Zoster Vaccine in Patients with
Inflammatory Bowel Disease. J Crohns Colitis 2022; 16(9): 1505-7.
239.
Satyam VR, Li PH, Reich J, et al. Safety of Recombinant Zoster Vaccine in Patients with Inflammatory Bowel
Disease. Dig Dis Sci 2020; 65(10): 2986-91.
79