よむ、つかう、まなぶ。
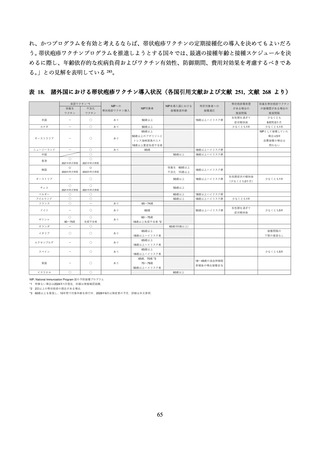
03 資料1-1 帯状疱疹ワクチンファクトシート (83 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_40826.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第26回 6/20)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
270.
The National Immunisation Advisory Committee (NIAC). NIAC Immunisation Guidelines. Chapter 23. Varicella
Zoster. 2022/10/21 updated 2022. https://rcpi.access.preservica.com/uncategorized/IO_83ed18c1-91c1-46ac-9fe4e29fc536a5d8/ (accessed 2024/04/26).
271.
Ministère du Travail, de la Santé et des Solidarités フランス労働保健省. Calendrier des vaccinations et
recommandations vaccinales 2023. 2023/06 2023. https://sante.gouv.fr/IMG/pdf/calendrier_vaccinal_maj-juin23.pdf
(accessed 2024/04/26).
272.
publique HCdls. Vaccination des adultes contre le zona avec le vaccin Zostavax®. 2013/12/11 2013.
https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=389 (accessed 2024/04/26).
273.
Siedler A, Koch J, Garbe E, et al. Background paper to the decision to recommend the vaccination with the
inactivated herpes zoster subunit vaccine : Statement of the German Standing Committee on Vaccination (STIKO) at
the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2019; 62(3): 352-76.
274.
National Health Care Institute. ZOSTAVAX for the prevention of herpes zoster and postherpetic neuralgia.
2013/09/17 2013. https://english.zorginstituutnederland.nl/publications/reports/2013/09/17/zostavax-for-theprevention-of-herpes-zoster-and-postherpetic-neuralgia (accessed 2024/04/26).
275.
Ceccarelli A, Tamarri F, Angelini R, et al. Herpes Zoster Vaccine Uptake and Active Campaign Impact, a
Multicenter Retrospective Study in Italy. Vaccines (Basel) 2024; 12(1).
276.
Risco Risco C, Herrador Z, Lopez-Perea N, Martínez-Urbistondo D, Del Villar Carrero RS, Masa-Calles J.
Epidemiology of Herpes Zoster in the pre-vaccination era: establishing the baseline for vaccination programme's
impact in Spain. Euro Surveill 2023; 28(8).
277.
Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation Statement
on varicella and herpes zoster vaccines. 2010.
https://webarchive.nationalarchives.gov.uk/ukgwa/20100407235417mp_/http://www.dh.gov.uk/prod_consum_dh/gr
oups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_114908.pdf (accessed 2024/04/26).
278.
van Oorschot DAM, Hunjan M, Bracke B, Lorenc S, Curran D, Starkie-Camejo H. Public health impact model
estimating the impact of introducing an adjuvanted recombinant zoster vaccine into the UK universal mass
vaccination programme. BMJ Open 2019; 9(5): e025553.
279.
GOV.UK. Guidance Shingles immunisation programme: information for healthcare practitioners. 2023/09/07
2023. https://www.gov.uk/government/publications/shingles-vaccination-guidance-for-healthcareprofessionals/shingles-immunisation-programme-information-for-healthcare-practitioners2024/04/26).
280.
GOV.UK. the green book, Chapter 28a: Shingles (herpes zoster). 2023/07/26 2023.
https://assets.publishing.service.gov.uk/media/64c1153cd4051a000d5a9409/Shingles_Green_Book_on_Immunisatio
n_Chapter_28a_26_7_23.pdf.
281.
Ministry of Health of Israel. What Vaccines Are Right For Me
Herpes Zoster Vaccine.
https://me.health.gov.il/en/older-adult/services-rights/vaccines-old-age/customized-vaccines/ (accessed 2024/03/01).
282.
Herpes zoster vaccines.
http://www.who.int/immunization/sage/meetings/2014/april/2_Background_document_Herpes_Zoster.pdf.
283.
Varicella and herpes zoster vaccines: WHO position paper, June 2014. http://www.who.int/wer/2014/wer8925/en/.
82
The National Immunisation Advisory Committee (NIAC). NIAC Immunisation Guidelines. Chapter 23. Varicella
Zoster. 2022/10/21 updated 2022. https://rcpi.access.preservica.com/uncategorized/IO_83ed18c1-91c1-46ac-9fe4e29fc536a5d8/ (accessed 2024/04/26).
271.
Ministère du Travail, de la Santé et des Solidarités フランス労働保健省. Calendrier des vaccinations et
recommandations vaccinales 2023. 2023/06 2023. https://sante.gouv.fr/IMG/pdf/calendrier_vaccinal_maj-juin23.pdf
(accessed 2024/04/26).
272.
publique HCdls. Vaccination des adultes contre le zona avec le vaccin Zostavax®. 2013/12/11 2013.
https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=389 (accessed 2024/04/26).
273.
Siedler A, Koch J, Garbe E, et al. Background paper to the decision to recommend the vaccination with the
inactivated herpes zoster subunit vaccine : Statement of the German Standing Committee on Vaccination (STIKO) at
the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2019; 62(3): 352-76.
274.
National Health Care Institute. ZOSTAVAX for the prevention of herpes zoster and postherpetic neuralgia.
2013/09/17 2013. https://english.zorginstituutnederland.nl/publications/reports/2013/09/17/zostavax-for-theprevention-of-herpes-zoster-and-postherpetic-neuralgia (accessed 2024/04/26).
275.
Ceccarelli A, Tamarri F, Angelini R, et al. Herpes Zoster Vaccine Uptake and Active Campaign Impact, a
Multicenter Retrospective Study in Italy. Vaccines (Basel) 2024; 12(1).
276.
Risco Risco C, Herrador Z, Lopez-Perea N, Martínez-Urbistondo D, Del Villar Carrero RS, Masa-Calles J.
Epidemiology of Herpes Zoster in the pre-vaccination era: establishing the baseline for vaccination programme's
impact in Spain. Euro Surveill 2023; 28(8).
277.
Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation Statement
on varicella and herpes zoster vaccines. 2010.
https://webarchive.nationalarchives.gov.uk/ukgwa/20100407235417mp_/http://www.dh.gov.uk/prod_consum_dh/gr
oups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_114908.pdf (accessed 2024/04/26).
278.
van Oorschot DAM, Hunjan M, Bracke B, Lorenc S, Curran D, Starkie-Camejo H. Public health impact model
estimating the impact of introducing an adjuvanted recombinant zoster vaccine into the UK universal mass
vaccination programme. BMJ Open 2019; 9(5): e025553.
279.
GOV.UK. Guidance Shingles immunisation programme: information for healthcare practitioners. 2023/09/07
2023. https://www.gov.uk/government/publications/shingles-vaccination-guidance-for-healthcareprofessionals/shingles-immunisation-programme-information-for-healthcare-practitioners2024/04/26).
280.
GOV.UK. the green book, Chapter 28a: Shingles (herpes zoster). 2023/07/26 2023.
https://assets.publishing.service.gov.uk/media/64c1153cd4051a000d5a9409/Shingles_Green_Book_on_Immunisatio
n_Chapter_28a_26_7_23.pdf.
281.
Ministry of Health of Israel. What Vaccines Are Right For Me
Herpes Zoster Vaccine.
https://me.health.gov.il/en/older-adult/services-rights/vaccines-old-age/customized-vaccines/ (accessed 2024/03/01).
282.
Herpes zoster vaccines.
http://www.who.int/immunization/sage/meetings/2014/april/2_Background_document_Herpes_Zoster.pdf.
283.
Varicella and herpes zoster vaccines: WHO position paper, June 2014. http://www.who.int/wer/2014/wer8925/en/.
82