よむ、つかう、まなぶ。
【参考資料6】抗微生物薬適正使用の手引き 第三版 補遺 (36 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_45318.html |
| 出典情報 | 厚生科学審議会 感染症部会 薬剤耐性(AMR)に関する小委員会 抗微生物薬適正使用(AMS)等に関する作業部会(第6回 11/19)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
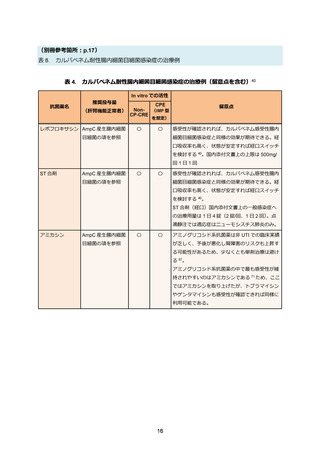
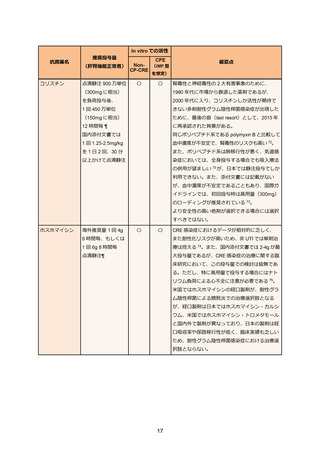
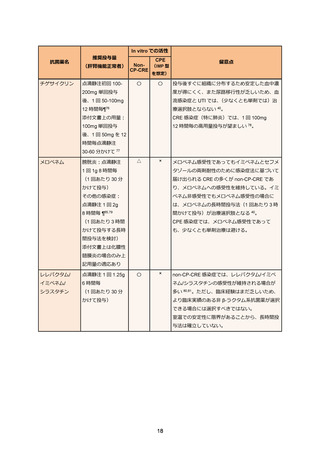
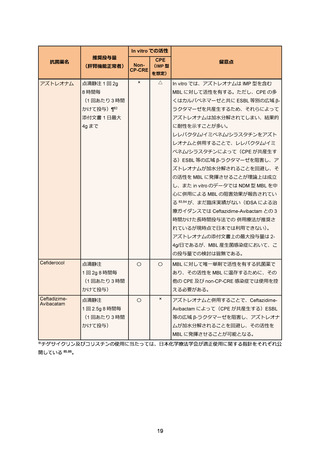
69. Bassetti M, Echols R, Matsunaga Y. et al. Efficacy and safety of cefiderocol or best available
therapy for the treatment of serious infections caused by carbapenem-resistant Gramnegative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogenfocused, descriptive, phase 3 trial. Lancet Infect Dis. 2021 Feb;21(2):226-240.
70. Timsit JF, Paul M, Shields RK. et al. Cefiderocol for the Treatment of Infections Due to
Metallo-B-lactamase-Producing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3
Randomized Studies. Clin Infect Dis. 2022 Sep;75(6):1081-1084.
71. Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. In Vitro Activity of
Plazomicin against Gram-Negative and Gram-Positive Isolates Collected from U.S. Hospitals
and Comparative Activities of Aminoglycosides against Carbapenem-Resistant
Enterobacteriaceae and Isolates Carrying Carbapenemase Genes. Antimicrob Agents
Chemother. 2018 Jul;62(8):e00313-18.
72. Vardakas KZ, Falagas ME. Colistin versus polymyxin B for the treatment of patients with
multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int J
Antimicrob Agents. 2017 Feb;49(2):233-238.
73. Tsuji BT, Pogue JM, Zavascki AP. et al. International Consensus Guidelines for the Optimal
Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP),
European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious
Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology
(ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases
Pharmacists (SIDP). Pharmacotherapy. 2019 Jan;39(1):10-39.
74. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev.
2016 Apr;29(2):321-347.
75. Sojo-Dorado J, Lopez-Hernandez I, Rosso-Fernandez C. et al. Effectiveness of Fosfomycin
for the Treatment of Multidrug-Resistant Escherichia coli Bacteremic Urinary Tract Infections:
A Randomized Clinical Trial. JAMA Netw Open. 2022 Jan;5(1):e2137277.
76. Zha L, Pan L, Guo J, French N, Villanueva EV, Tefsen B. Effectiveness and Safety of High
Dose Tigecycline for the Treatment of Severe Infections: A Systematic Review and MetaAnalysis. Adv Ther. 2020 Mar;37(3):1049-1064.
77. De Pascale G, Lisi L, Ciotti GMP. et al. Pharmacokinetics of high-dose tigecycline in critically
ill patients with severe infections. Ann Intensive Care. 2020 Jul;10(1):94.
78. Ni W, Han Y, Liu J. et al. Tigecycline Treatment for Carbapenem-Resistant
Enterobacteriaceae Infections: A Systematic Review and Meta-Analysis. Medicine
(Baltimore). 2016 Mar;95(11):e3126.
79. Pascale R, Giannella M, Bartoletti M, Viale P, Pea F. Use of meropenem in treating
carbapenem-resistant Enterobacteriaceae infections. Expert Rev Anti Infect Ther. 2019
Oct;17(10):819-827.
80. Bonnin RA, Bernabeu S, Emeraud C. et al. In Vitro Activity of Imipenem-Relebactam,
Meropenem-Vaborbactam, Ceftazidime-Avibactam and Comparators on CarbapenemResistant Non-Carbapenemase-Producing Enterobacterales. Antibiotics (Basel). 2023
Jan;12(1):102.
36
therapy for the treatment of serious infections caused by carbapenem-resistant Gramnegative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogenfocused, descriptive, phase 3 trial. Lancet Infect Dis. 2021 Feb;21(2):226-240.
70. Timsit JF, Paul M, Shields RK. et al. Cefiderocol for the Treatment of Infections Due to
Metallo-B-lactamase-Producing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3
Randomized Studies. Clin Infect Dis. 2022 Sep;75(6):1081-1084.
71. Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. In Vitro Activity of
Plazomicin against Gram-Negative and Gram-Positive Isolates Collected from U.S. Hospitals
and Comparative Activities of Aminoglycosides against Carbapenem-Resistant
Enterobacteriaceae and Isolates Carrying Carbapenemase Genes. Antimicrob Agents
Chemother. 2018 Jul;62(8):e00313-18.
72. Vardakas KZ, Falagas ME. Colistin versus polymyxin B for the treatment of patients with
multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int J
Antimicrob Agents. 2017 Feb;49(2):233-238.
73. Tsuji BT, Pogue JM, Zavascki AP. et al. International Consensus Guidelines for the Optimal
Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP),
European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious
Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology
(ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases
Pharmacists (SIDP). Pharmacotherapy. 2019 Jan;39(1):10-39.
74. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev.
2016 Apr;29(2):321-347.
75. Sojo-Dorado J, Lopez-Hernandez I, Rosso-Fernandez C. et al. Effectiveness of Fosfomycin
for the Treatment of Multidrug-Resistant Escherichia coli Bacteremic Urinary Tract Infections:
A Randomized Clinical Trial. JAMA Netw Open. 2022 Jan;5(1):e2137277.
76. Zha L, Pan L, Guo J, French N, Villanueva EV, Tefsen B. Effectiveness and Safety of High
Dose Tigecycline for the Treatment of Severe Infections: A Systematic Review and MetaAnalysis. Adv Ther. 2020 Mar;37(3):1049-1064.
77. De Pascale G, Lisi L, Ciotti GMP. et al. Pharmacokinetics of high-dose tigecycline in critically
ill patients with severe infections. Ann Intensive Care. 2020 Jul;10(1):94.
78. Ni W, Han Y, Liu J. et al. Tigecycline Treatment for Carbapenem-Resistant
Enterobacteriaceae Infections: A Systematic Review and Meta-Analysis. Medicine
(Baltimore). 2016 Mar;95(11):e3126.
79. Pascale R, Giannella M, Bartoletti M, Viale P, Pea F. Use of meropenem in treating
carbapenem-resistant Enterobacteriaceae infections. Expert Rev Anti Infect Ther. 2019
Oct;17(10):819-827.
80. Bonnin RA, Bernabeu S, Emeraud C. et al. In Vitro Activity of Imipenem-Relebactam,
Meropenem-Vaborbactam, Ceftazidime-Avibactam and Comparators on CarbapenemResistant Non-Carbapenemase-Producing Enterobacterales. Antibiotics (Basel). 2023
Jan;12(1):102.
36