よむ、つかう、まなぶ。
【参考資料6】抗微生物薬適正使用の手引き 第三版 補遺 (37 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_45318.html |
| 出典情報 | 厚生科学審議会 感染症部会 薬剤耐性(AMR)に関する小委員会 抗微生物薬適正使用(AMS)等に関する作業部会(第6回 11/19)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
81. Senchyna F, Gaur RL, Sandlund J. et al. Diversity of resistance mechanisms in carbapenemresistant Enterobacteriaceae at a health care system in Northern California, from 2013 to
2016. Diagn Microbiol Infect Dis. 2019 Mar;93(3):250-257.
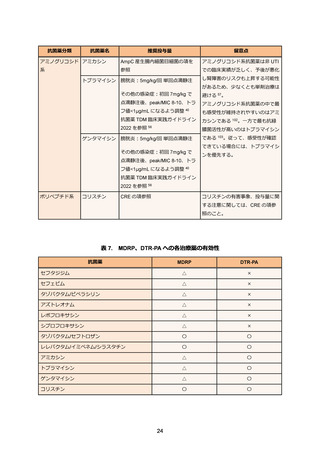
82. Vinks AA, van Rossem RN, Mathot RA, Heijerman HG, Mouton JW. Pharmacokinetics of
aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of doseexposure relationships using monte carlo simulation. Antimicrob Agents Chemother. 2007
Sep;51(9):3049-3055.
83. Biagi M, Lee M, Wu T. et al. Aztreonam in combination with imipenem-relebactam against
clinical and isogenic strains of serine and metallo-beta-lactamase-producing
enterobacterales. Diagn Microbiol Infect Dis. 2022 Jun;103:115674.
84. Maraki S, Mavromanolaki VE, Moraitis P. et al. Ceftazidime-avibactam, meropenenvaborbactam, and imipenem-relebactam in combination with aztreonam against multidrugresistant, metallo-beta-lactamase-producing Klebsiella pneumoniae. Eur J Clin Microbiol
Infect Dis. 2021 Aug;40(8):1755-1759.
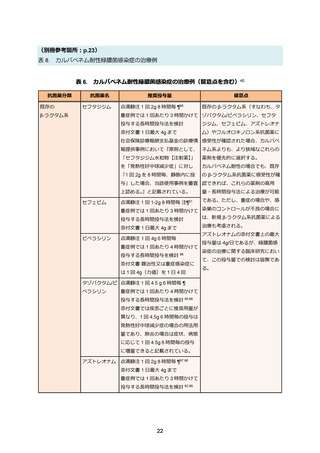
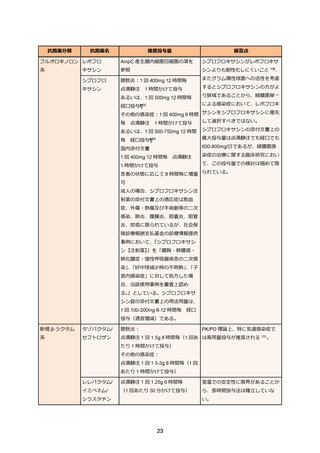
85. コリスチンの適正使用に関する指針―改訂版―, 日本化学療法学会. 2015. at
https://www.chemotherapy.or.jp/uploads/files/guideline/colistin_guideline_update.pdf.)
86. チゲサイクリン適正使用のための手引き 2014 日本化学療法学会雑誌. 2014;62:311-66.
87. M100-32nd Edition. at http://em100.edaptivedocs.net/dashboard.aspx,.)
88. Mano Y, Saga T, Ishii Y. et al. Molecular analysis of the integrons of metallo-beta-lactamaseproducing Pseudomonas aeruginosa isolates collected by nationwide surveillance programs
across Japan. BMC Microbiol. 2015 Feb;15:41.
89. Hishinuma T, Uchida H, Tohya M, Shimojima M, Tada T, Kirikae T. Emergence and spread of
VIM-type metallo-beta-lactamase-producing Pseudomonas aeruginosa clinical isolates in
Japan. J Glob Antimicrob Resist. 2020 Dec;23:265-268.
90. Hishinuma T, Tada T, Kuwahara-Arai K, Yamamoto N, Shimojima M, Kirikae T. Spread of
GES-5 carbapenemase-producing Pseudomonas aeruginosa clinical isolates in Japan due to
clonal expansion of ST235. PLoS One. 2018 Nov;13(11):e0207134.
91. Pogue JM, Kaye KS, Veve MP. et al. Ceftolozane/Tazobactam vs Polymyxin or
Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas
aeruginosa. Clin Infect Dis. 2020 Jul;71(2):304-310.
92. Almangour TA, Aljabri A, Al Musawa M. et al. Ceftolozane-tazobactam vs. colistin for the
treatment of infections due to multidrug-resistant Pseudomonas aeruginosa: a multicentre
cohort study. J Glob Antimicrob Resist. 2022 Mar;28:288-294.
93. Rebold N, Morrisette T, Lagnf AM. et al. Early Multicenter Experience With ImipenemCilastatin-Relebactam for Multidrug-Resistant Gram-Negative Infections. Open Forum Infect
Dis. 2021 Dec;8(12):ofab554.
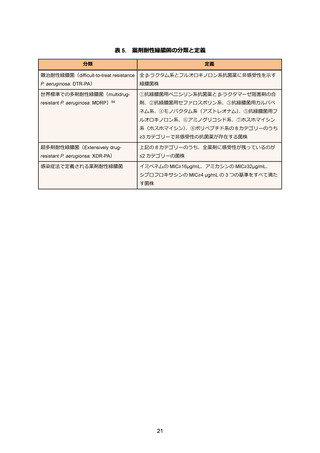
94. Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant
and pandrug-resistant bacteria: an international expert proposal for interim standard
definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-281.
37
2016. Diagn Microbiol Infect Dis. 2019 Mar;93(3):250-257.
82. Vinks AA, van Rossem RN, Mathot RA, Heijerman HG, Mouton JW. Pharmacokinetics of
aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of doseexposure relationships using monte carlo simulation. Antimicrob Agents Chemother. 2007
Sep;51(9):3049-3055.
83. Biagi M, Lee M, Wu T. et al. Aztreonam in combination with imipenem-relebactam against
clinical and isogenic strains of serine and metallo-beta-lactamase-producing
enterobacterales. Diagn Microbiol Infect Dis. 2022 Jun;103:115674.
84. Maraki S, Mavromanolaki VE, Moraitis P. et al. Ceftazidime-avibactam, meropenenvaborbactam, and imipenem-relebactam in combination with aztreonam against multidrugresistant, metallo-beta-lactamase-producing Klebsiella pneumoniae. Eur J Clin Microbiol
Infect Dis. 2021 Aug;40(8):1755-1759.
85. コリスチンの適正使用に関する指針―改訂版―, 日本化学療法学会. 2015. at
https://www.chemotherapy.or.jp/uploads/files/guideline/colistin_guideline_update.pdf.)
86. チゲサイクリン適正使用のための手引き 2014 日本化学療法学会雑誌. 2014;62:311-66.
87. M100-32nd Edition. at http://em100.edaptivedocs.net/dashboard.aspx,.)
88. Mano Y, Saga T, Ishii Y. et al. Molecular analysis of the integrons of metallo-beta-lactamaseproducing Pseudomonas aeruginosa isolates collected by nationwide surveillance programs
across Japan. BMC Microbiol. 2015 Feb;15:41.
89. Hishinuma T, Uchida H, Tohya M, Shimojima M, Tada T, Kirikae T. Emergence and spread of
VIM-type metallo-beta-lactamase-producing Pseudomonas aeruginosa clinical isolates in
Japan. J Glob Antimicrob Resist. 2020 Dec;23:265-268.
90. Hishinuma T, Tada T, Kuwahara-Arai K, Yamamoto N, Shimojima M, Kirikae T. Spread of
GES-5 carbapenemase-producing Pseudomonas aeruginosa clinical isolates in Japan due to
clonal expansion of ST235. PLoS One. 2018 Nov;13(11):e0207134.
91. Pogue JM, Kaye KS, Veve MP. et al. Ceftolozane/Tazobactam vs Polymyxin or
Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas
aeruginosa. Clin Infect Dis. 2020 Jul;71(2):304-310.
92. Almangour TA, Aljabri A, Al Musawa M. et al. Ceftolozane-tazobactam vs. colistin for the
treatment of infections due to multidrug-resistant Pseudomonas aeruginosa: a multicentre
cohort study. J Glob Antimicrob Resist. 2022 Mar;28:288-294.
93. Rebold N, Morrisette T, Lagnf AM. et al. Early Multicenter Experience With ImipenemCilastatin-Relebactam for Multidrug-Resistant Gram-Negative Infections. Open Forum Infect
Dis. 2021 Dec;8(12):ofab554.
94. Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant
and pandrug-resistant bacteria: an international expert proposal for interim standard
definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-281.
37