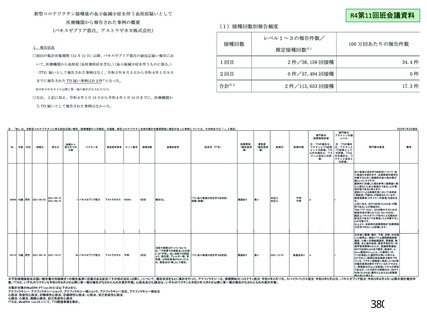
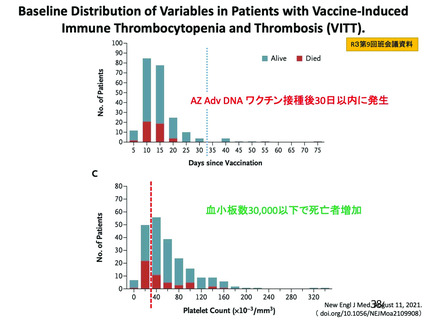
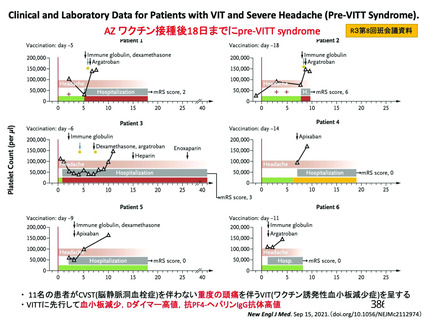
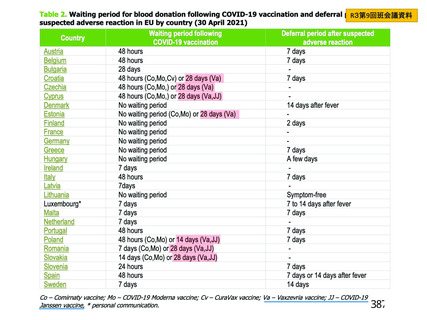
資 料4-2-➁ 令和4年度第2回安全技術調査会の審議結果について➁ (118 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27906.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会血液事業部会(令和4年度第2回 9/14)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
IPFA position on acceptance criteria for CovId-
19 vaccinated donors
2 October 2021
Dr Francoise RoSSi,
Director of Scientific and Regulatory Affairs
First published Jan 2021, Rev. July 2021
IPFA position on acceptance Criteria for Covid-19 vaccinated donors
In anticipation of the regulatory approval for use of a number of Covid-19 vaccines
and the commencement of national mass vaccination proqrammes the European
Centre for Disease Prevention and Control (ECDC) has published its updated
technical guidance - Coronavirus disease 2019 (COVID-19) and supply of
substances of human origin in the EU/EEA - second update.
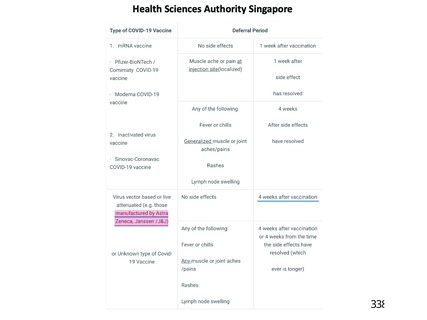
The guidance in respect of donor deferral following vaccination recommends:
s* Aminimum deferral of 4 weeks for investigational vaccines (clinical trials) of
any type
* Nodeferral period for mRNA or protein vaccines
s* Aminimum of 4 weeks for viral vector-type vaccines when considered
“attenuated virus” (as per Directive 2004/33).
https://wwwr.ecdc.europa.eu/sit ef lles/do
substances-human-origin-second-update.pdf
ents/covid-19-supply-
On Dec 12th, 2020, the PEI published its recommendation concerninq Dost
vaccination donor deferral in line with the above stating that:
“O7 妨e pgs/S O7 妨e Cu/7e77 S7g76 O7 7OW/eOe, 7O OO/ e7677g/ /S /07/760 g7e/
gcC787O7 M7訪 妨e S4RS-Co/2 vgco/7eS 7のe/ gpP/OVg/ MC Co7如
8CがV37@9 V/7USeS O7 7の7-776CがOS V770S CO77DO7677な SC gs MA. 47 o娘er
ge記gu/7 c77fe7g se7 ouf 7 妨e 右e77o妨egpy の77ecがve /e77877 7 gpp/cagの/e. "(in
German: https:/www.pei.de/EN/medicine-
safety/haemovigilance/guidelines/quidelines-
node.htmliisessionid=12DF32D0B1D6547F1004A53F0749348D.intranet211 )
On January 19, 2021, FDA published an Updated Information for Blood
Establishments Regarding the COVID-19 Pandemic and Blood Donation
recommending
s individuals who received a nonreplicatina, inactivated, or mRNA-based COVID-
19 vaccine can donate blood without a waiting Deriod,
s jindividuals who received a live-attenuated viral COVID-19 vaccine, refrain from
donatinq blood for a short waiting period (e.q., 14 days) after receipt of the
vaccine
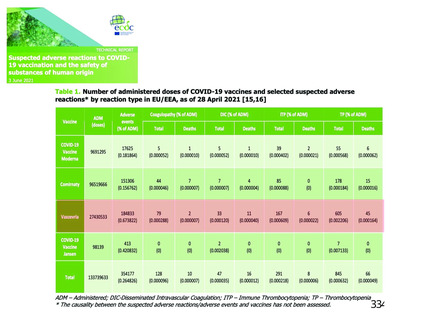
On 3 Jun 2021, the ECDC published a report on Suspected adverse reactions to
COVID-19 vaccination and the safety of substances of human origin, stating that
"CO7@77がy 23378の/6 279 27 ee/7Ce SUの96S7 9 /OW p/Oめagめ廊Of M力o/e の/Oo 27び
ps7778 ogがO7 のy 2Sy7D7O77gがC 7のuag/S 太 妨e eg/ル pse 07 7 7S poS/7の9
Ve /OW /7S ove7ep077C7/7e の/eed779 の7 OSたが37S娘S7O7 妨/O77のOcy7ODe77/8 のy
2SS/Ve か27S767 の7 7がり/g7e/e7 277がのOO7es. 7e/e7o/@, の 397が7g7 の/OO0 27の
pgs7773 Seが /763S07GS /@/g7e 7の 妨e OCCu/7e/C@ O7 SUSDeC7G ge/S@ /gCが7O77S
7o COW//-79 gcc/7eS 8/@ /@CO/77777677eの “
Whilst IPFA stronqly supports the above current recommendations it iS also
important to recognise the impact on the global blood and plasma supply of the
Covid pandemic and accordinaqly advocates caution in the development of any
future requlatory actions, based on the precautionary principle, concerning donor
deferral which may further worsen plasma collection and consequently Plasma
derived medicinal products suppIy.
326