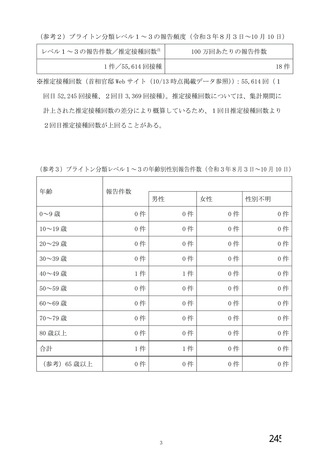
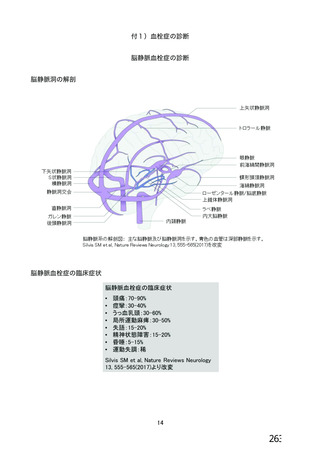
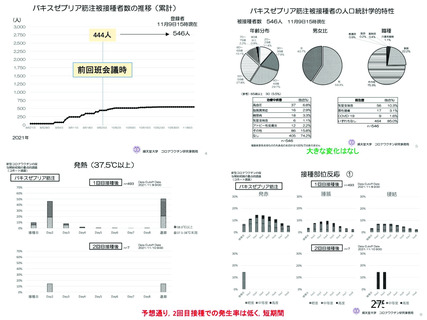
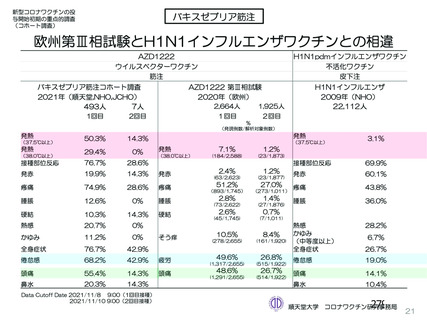
資 料4-2-➁ 令和4年度第2回安全技術調査会の審議結果について➁ (52 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27906.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会血液事業部会(令和4年度第2回 9/14)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg. 2017; 9:
1086-1092. doi: 10.1136/neurintsurg-2016-012938.
37. Coutinho JM, Zuurbier SM, Bousser MG, Ji X, Canhão P, Roos YB, Crassard I, Nunes AP, Uyttenboogaart M,
Chen J, Emmer BJ, Roosendaal SD, Houdart E, Reekers JA, van den Berg R, de Haan RJ, Majoie CB, Ferro
JM, Stam J; TO-ACT investigators. Effect of Endovascular Treatment With Medical Management vs
Standard Care on Severe Cerebral Venous Thrombosis: The TO-ACT Randomized Clinical Trial. JAMA
Neurol. 2020; 77: 966-973. doi: 10.1001/jamaneurol.2020.1022.
38. Alaraj A, Wallace A, Tesoro E, Ruland S, Amin-Hanjani S, Charbel FT, Aletich V. Heparin induced
thrombocytopenia: diagnosis and management. J Neurointerv Surg. 2010; 2: 371-378. doi:
10.1136/jnis.2010.002840.
39. Alaraj A, Tobin M, Birk D, Aletich V. Role of argatroban during neurointerventional procedures in
patients with heparin induced thrombocytopenia. J Neurointerv Surg. 2014; 6: 630-632. doi:
10.1136/neurintsurg-2013-010712.
40. Théaudin M, Crassard I, Bresson D, Saliou G, Favrole P, Vahedi K, Denier C, Bousser MG. Should
decompressive surgery be performed in malignant cerebral venous thrombosis?: a series of 12 patients.
Stroke. 2010; 41: 727-731. doi: 10.1161/STROKEAHA.109.572909.
41. Aaron S, Alexander M, Moorthy RK, Mani S, Mathew V, Patil AK, Sivadasan A, Nair S, Joseph M, Thomas
M, Prabhu K, Joseph BV, Rajshekhar V, Chacko AG. Decompressive craniectomy in cerebral venous
thrombosis: a single centre experience. J Neurol Neurosurg Psychiatry. 2013; 84: 995-1000. doi:
10.1136/jnnp-2012-303356.
42. Ferro JM, Crassard I, Coutinho JM, Canhão P, Barinagarrementeria F, Cucchiara B, Derex L, Lichy C,
Masjuan J, Massaro A, Matamala G, Poli S, Saadatnia M, Stolz E, Viana-Baptista M, Stam J, Bousser MG;
Second International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT 2) Investigators:
Decompressive surgery in cerebrovenous thrombosis: a multicenter registry and a systematic review of
individual patient data. Stroke. 2011; 42: 2825-2831. doi: 10.1161/STROKEAHA.111.615393.
43. Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I,
Masuhr F, Aguiar de Sousa D, Stam J; European Stroke Organization. European Stroke Organization
guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European
Academy of Neurology. Eur J Neurol. 2017; 24: 1203-1213. doi: 10.1111/ene.13381.
44. Davoudi V, Keyhanian K, Saadatnia M. Risk factors for remote seizure development in patients with
cerebral
vein
and
dural
sinus
thrombosis.
Seizure.
2014;
23:
135-139.
doi:
10.1016/j.seizure.2013.10.011.
45. Price M, Günther A, Kwan JSK. Antiepileptic drugs for the primary and secondary prevention of seizures
after intracranial venous thrombosis. Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD005501. doi:
10.1002/14651858.CD005501.pub4.
21
270