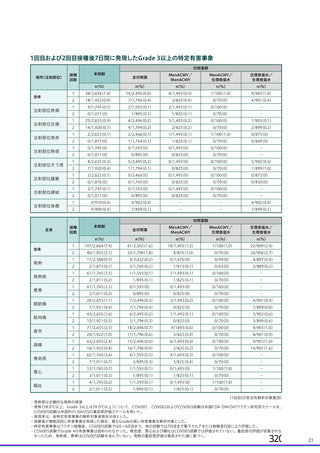
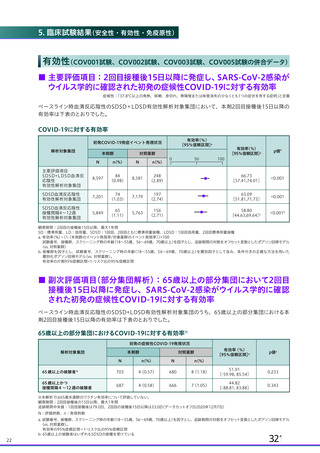
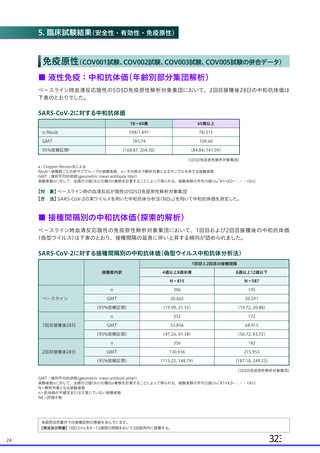
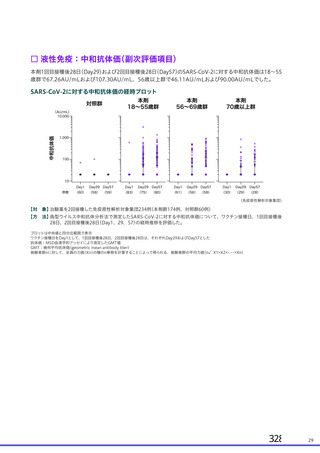
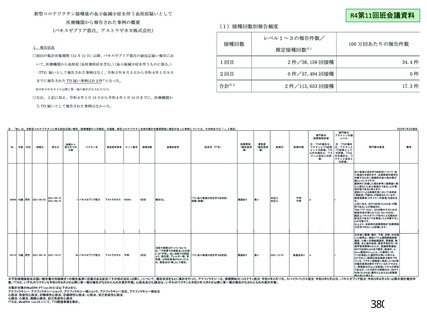
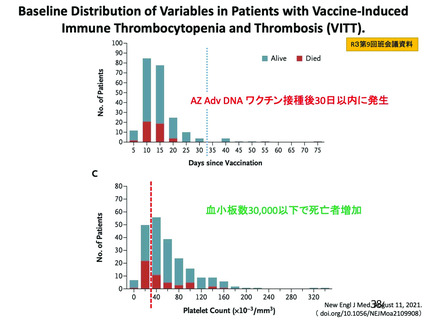
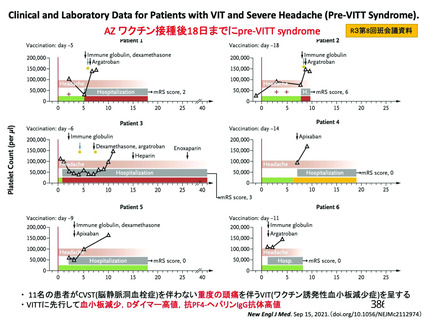
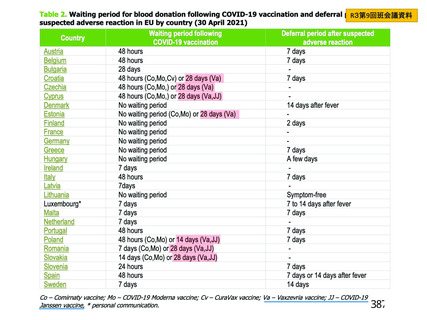
資 料4-2-➁ 令和4年度第2回安全技術調査会の審議結果について➁ (119 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27906.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会血液事業部会(令和4年度第2回 9/14)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
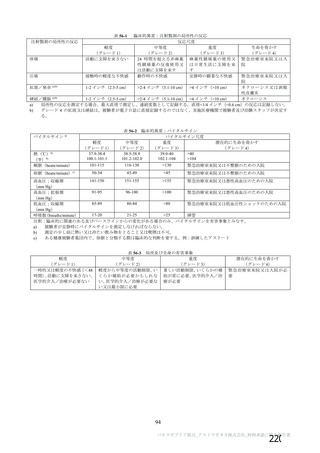
Implementation: To be determined by each Service
Chandge Notification UK National Blood Services No. 11 - 2021
Op/9a7oy:
り/Scye7o787:
Agg77o7g/ oy77787がOn:
a) Recipients of a COVID-19 vaccine in the UK vaccination programme
Must not donate if:
) Less than 14 seyen days after the last immunization was-diveh if the
vaccine given was nucleic acid (mRNA) vaccine.
持必donor fe unwell after vacclnatlon。 mustnot donate_for 7 days aftefr
i Less than 28 days after the last immunization if the vaccine given was
See additional information for further information on different types of vaccine.
員) lf donor felt unwell due to unexpected complications (other than common
side effects) after any vaccination, nusLnetLdenate-for-
efsyfBtems. refer to Desiqgnated Clinical Support Officer for individual risk
assessment.
Timings above refer to interval between vaccination and start of G-CSF or
general anaesthetic for BM donation.
b) Recipients of a COVID-19 vaccine outside the UK vaccination
program, including participants in clinical trials or donors vaccinated
outside the UK
Refer to Designated Clinical Support Officer for individual risk assessment.
See additional information.
lf the transplant cannot be delayed. Donors may be accepted less than 14了
days (nucleic acid vaccines) or 28 days (viral vector vaccines) after the date
of the most recent vaccination. if vaccinated as part of the UK vaccination
proqramme, Subject to individual risk assessment. See additional information.
0人
default be deferred for four weeKS.
All COVID-19 vaccines currently licensed in the UK are non-live. Normally.
no deferral period is applied after immunisation with non-live vaccines.
However as the effects of the newly developed coronavirus vaccines on
donor health and donation safety are not fully established yet, as a
precautionary Drinciple, a day 14 to 28 day post vaccine deferral period、
valak on 0 DD の Vaccine 人本FM
0 IS PSGOYHmGhSHed
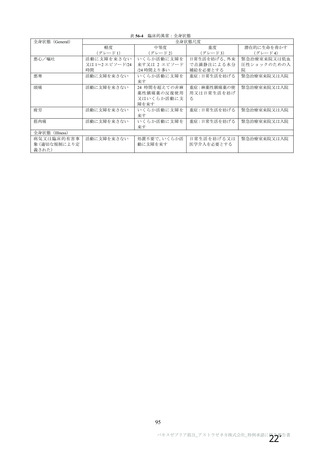
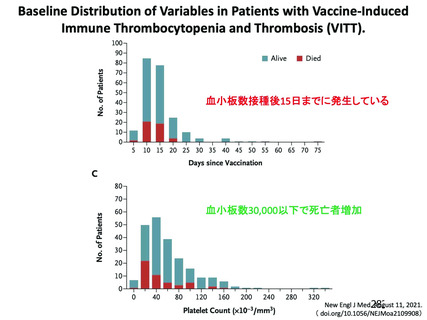
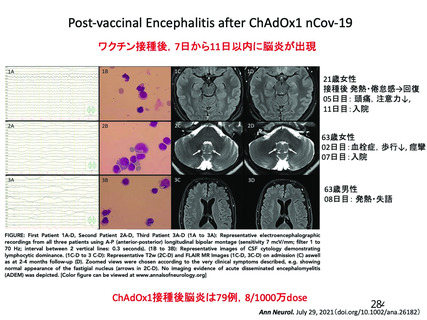
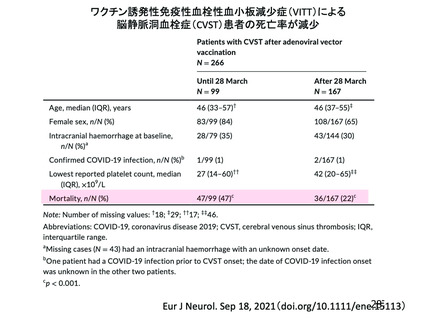
Immune thrombocytopenia (ITP) can occur after all types of Covid 19
vaccines. There have been a small number of reports of vaccine Induced
thrombosis and thrombocytopenia syndrome (VITTS), in people receiving
virus vector based (non-replicating) coronavirus vaccine. VITTS patients
have severe clinical symptoms whilst ITP may be sub-clinical and go
unnoticed on symptoms alone. The incidence iS unclear but may be similar
to other vaccine induced ITP. Therefore a 14 day deferral period has been
recommended after vaccination with mRNA vaccineS.
Appendix 3. Table of Immunizations
Diseases Protected against
GCSF administration carries a small riSk of inflammation associated
thrombosis and thrombocytopenia. There is a theoretical concern that
GCSF could exacerbate the immune response related to VITTS. Headaches
and abdominal pain are side effects of GCSF which are primary Symptoms
assoclated with cerebral venous thrombosis and splanchnic vein thrombosis
respectively, due to VITTS. As a precautionary measure the post
vaccination deferral period for bone marrow and PBSC donors receivind
virus-vector-based (non-replicating virus) vaccines has been extended to 28
dayS, for donor protection. As the reported events are extremely rare,
donors may be accepted less than 28 days after vaccination subject to a
careful individualised risk assessment.
Consideration of checking a platelet count after vaccination to rule out
{hrombocytopenia is recommended. This could be included as a part of
medical assessment if undertaken 14 days or more after vaccination. lf less
than 14 days between vaccination and medical assessment, or vaccination
was given after medical assessment, additional Full Blood Count should be
done before commencing GCSF/ general anaesthetic (frozen cells) and
before commencing patient conditioning (for fresh cells).
For donors who have commenced GCSF. the vaccination (first or second
dose) must be delayed at least until 72 hours after stem cell collection (both
PBSC & Bone Marrow Donation). This is a precautionary advice to avoid
vaccination when receiving GCSF and allow for post donation recovery
period.
EMRGteeue-gnd-GelLdonere rmmbAveeakoleotr eveeeweneeY fay be
dsublect to ahdhvtdhklaL fnSネ しせ4he beneftG「 内
laneplant-outwetghe-the-fieke-Of-dontion
For donors vaccinated as part of a clinical trial or outside of the UK. the type
of vaccine used should be established to determine the appropriate deferral
period.
There may be new types of vaccine that become available. and it may not
be known which type of vaccine was used for immunisation. In situations
where information about vaccine type iS miSSing Or he vaccination iS
experimental. a four-week deferral period should be applied.
The British Society for Immunology has published an infographic to explain
to the general public the different types of COVID-19 vaccines, including
brand names, available in the UK. in other countries, and in Clinical trialS.
See the following link: https:/www.immunoloqy. org/coronavirus/connect
naviruS-DubDlic-en ment-r 『 { -V -f Vid- 1
The ECDC recofnmeftds hatEHSC dohors haye BeeR vaCeated wtth
attenuated vaccineS In he four weeks before donalion、a rlSk assesSftGnt
should be carrted out and 3ken itO account when deckhng On
tfansblartatoft and. 半 ansBlanted te fectDlent Shoultd be tonttofed DoSに
tranSDlant
Reason 7or Cカange: R f to fic brands of va - To increase the post
vaccination deferral period for nucleic acid (mRNA) vaccines to 14 days and
Virus-vector-based vaccines (non-replicating) to 28 days for donor
protection. Additional Information section has been updated.
Please make the following amendment to this table:
Comments and example trade names of adult
preparations
COVID-19 (SARS-CoV-2) PfizerBioNTech COVID-19 vaccine, う33 Non-Live
AstraZeneca COVID-19 vaccine, Moderna
COVID-19 vaccine了days_BostHimmmunlsatlon: See
*Coronavirus vaccination' entry