よむ、つかう、まなぶ。
資料3-1 リツキシマブ(遺伝子組換え) (50 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00026.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第54回 2/15)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
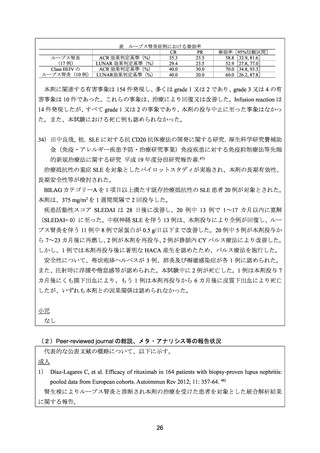
3) Terrier B, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from
136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:
2458-66.
4) Merrill JT, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic
lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus
evaluation of rituximab. Arthritis Rheum 2010; 62: 222-33.
5) Rovin BH, et al. Effect of rituximab (RTX) on anti-dsDNA and C3 levels and relationship to
response: results from the LUNAR trial. J Am Soc Nephrol 2009; 20: 406A.
6) Fanouriakis A, et al. 2019 update of the Joint European League Against Rheumatism and
European Renal Association-European Dialysis and Transplant Association (EULAR/
ERA-EDTA) recommendations for management of lupus nephritis. Ann Rheum Dis 2020; 79:
713-23.
7) Fanouriakis A, et al. 2019 update of the EULAR recommendations for the management of
systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736-45.
8) Groot N, et al. European evidence-based recommendations for the diagnosis and treatment of
childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 2017; 76: 1965-73.
9) Alshaiki, et al. Outcomes of rituximab therapy in refractory lupus: A meta-analysis. Eur J
Rheumatol 2018; 5: 118-26.
10) Weidenbusch M, et al. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus
nephritis. Nephrol Dial Transplant 2013; 28: 106-11.
11) Davies RJ, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly
progressive crescentic lupus nephritis. Lupus 2013; 22: 574-82.
12) Jónsdóttir T, et al. Long-term follow-up in lupus nephritis patientstreated with rituximab-clinical
and histopathological response. Rheumatology 2013; 52: 847-55.
13) Zhang J, et al. Effect of Rituximab on Serum Levels of Anti-C1q and Antineutrophil
Cytoplasmic Autoantibodies in Refractory Severe Lupus Nephritis. Cell Biochem Biophys 2015;
72:197-201.
14) Gomez Mendez LM, et al. Peripheral Blood B Cell Depletion after Rituximab and Complete
Response in Lupus Nephritis. Clin J Am Soc Nephrol 2018; 13: 1502-9.
15) McCarthy EM, et al. Short-term efficacy and safety of rituximab therapy in refractory systemic
lupus erythematosus: results from the British Isles Lupus Assessment Group Biologics Register.
Rheumatology 2018; 57: 470-9.
16) Fernández-Nebro A, et al. Multicenter longitudinal study of B-lymphocyte depletion in
refractory systemic lupus erythematosus: the LESIMAB study. Lupus 2012; 21: 1063-76.
17) Kraaij T, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus
erythematosus. J Autoimmun 2018; 91: 45-54.
18) Díaz-Lagares C, et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis:
50
136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:
2458-66.
4) Merrill JT, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic
lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus
evaluation of rituximab. Arthritis Rheum 2010; 62: 222-33.
5) Rovin BH, et al. Effect of rituximab (RTX) on anti-dsDNA and C3 levels and relationship to
response: results from the LUNAR trial. J Am Soc Nephrol 2009; 20: 406A.
6) Fanouriakis A, et al. 2019 update of the Joint European League Against Rheumatism and
European Renal Association-European Dialysis and Transplant Association (EULAR/
ERA-EDTA) recommendations for management of lupus nephritis. Ann Rheum Dis 2020; 79:
713-23.
7) Fanouriakis A, et al. 2019 update of the EULAR recommendations for the management of
systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736-45.
8) Groot N, et al. European evidence-based recommendations for the diagnosis and treatment of
childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 2017; 76: 1965-73.
9) Alshaiki, et al. Outcomes of rituximab therapy in refractory lupus: A meta-analysis. Eur J
Rheumatol 2018; 5: 118-26.
10) Weidenbusch M, et al. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus
nephritis. Nephrol Dial Transplant 2013; 28: 106-11.
11) Davies RJ, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly
progressive crescentic lupus nephritis. Lupus 2013; 22: 574-82.
12) Jónsdóttir T, et al. Long-term follow-up in lupus nephritis patientstreated with rituximab-clinical
and histopathological response. Rheumatology 2013; 52: 847-55.
13) Zhang J, et al. Effect of Rituximab on Serum Levels of Anti-C1q and Antineutrophil
Cytoplasmic Autoantibodies in Refractory Severe Lupus Nephritis. Cell Biochem Biophys 2015;
72:197-201.
14) Gomez Mendez LM, et al. Peripheral Blood B Cell Depletion after Rituximab and Complete
Response in Lupus Nephritis. Clin J Am Soc Nephrol 2018; 13: 1502-9.
15) McCarthy EM, et al. Short-term efficacy and safety of rituximab therapy in refractory systemic
lupus erythematosus: results from the British Isles Lupus Assessment Group Biologics Register.
Rheumatology 2018; 57: 470-9.
16) Fernández-Nebro A, et al. Multicenter longitudinal study of B-lymphocyte depletion in
refractory systemic lupus erythematosus: the LESIMAB study. Lupus 2012; 21: 1063-76.
17) Kraaij T, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus
erythematosus. J Autoimmun 2018; 91: 45-54.
18) Díaz-Lagares C, et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis:
50