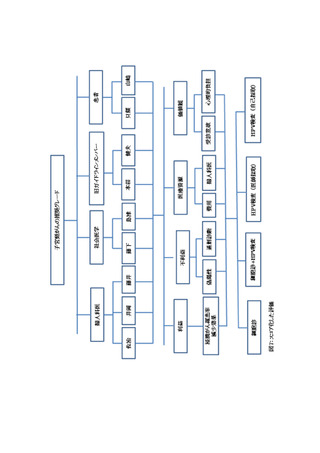
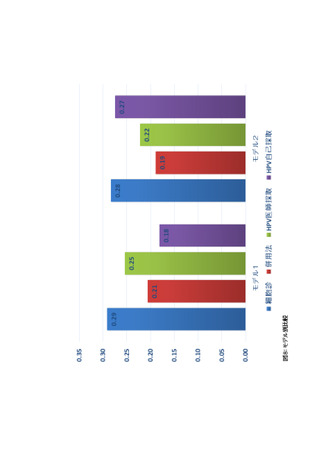
よむ、つかう、まなぶ。
参考資料4 有効性評価に基づく子宮頸がん検診ガイドライン更新版2020年3月31日 (65 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_25869.html |
| 出典情報 | がん検診のあり方に関する検討会(第35回 5/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
63) Jimenez-Perez M, Thomas DB. Has the use of Pap smears reduced the risk of
invasive cervical cancer in Guadalajara, Mexico? Int J Cancer. 1999; 82(6): 804-809.
64) Crocetti E, Battisti L, Betta A, Palma PD, Paci E, Piffer S, Pojer A, Polla E, Zappa
M. The cytological screening turned out effective also for adenocarcinoma: a populationbased case-control study in Trento, Italy. Eur J Cancer Prev. 2007; 16(6): 564-567.
65) Kasinpila C, Promthet S, Vatanasapt P, Sasieni P, Parkin DM. Evaluation of the
nationwide cervical screening programme in Thailand: a case-control study. J Med
Screen. 2011; 18(3): 147-153.
66) Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, Cheung LC,
Raine-Bennett TR, Gage JC, Kinney WK. Benchmarking CIN 3+ risk as the basis for
incorporating HPV and Pap cotesting into cervical screening and management
guidelines. J Low Genit Tract Dis. 2013; 17(5 Suppl 1): S28-35.
67) Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Robertson M, Castle PE; New Mexico
HPV Pap Registry Steering Committee. The influence of type-specific human
papillomavirus infections on the detection of cervical precancer and cancer: a populationbased study of opportunistic cervical screening in the United States. Int J Cancer. 2014;
135(3): 624-634.
68) Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, de Sanjose S,
Naucler P, Lloveras B, Kjaer S, Cuzick J, van Ballegooijen M, Clavel C, Iftner T. Long
term predictive values of cytology and human papillomavirus testing in cervical cancer
screening: joint European cohort study. BMJ. 2008; 337: a1754.
69) Arbyn M, Roelens J, Simoens C, Buntinx F, Paraskevaidis E, Martin-Hirsch PP,
Prendiville WJ. Human papillomavirus testing versus repeat cytology for triage of minor
cytological cervical lesions. Cochrane Database Syst Rev. 2013; 3: CD008054.
70) Elfström KM, Smelov V, Johansson AL, Eklund C, Nauclér P, Arnheim-Dahlström L,
Dillner J. Long term duration of protective effect for HPV negative women: follow-up of
primary HPV screening randomised controlled trial. BMJ. 2014; 348: g130.
71) Smelov V, Elfström KM, Johansson AL, Eklund C, Naucler P, Arnheim-Dahlström L,
Dillner J. Long-term HPV type-specific risks of high-grade cervical intraepithelial
lesions: a 14-year follow-up of a randomized primary HPV screening trial. Int J Cancer.
2015; 136(5): 1171-1180.
72) Dijkstra MG, van Zummeren M, Rozendaal L, van Kemenade FJ, Helmerhorst TJ,
Snijders PJ, Meijer CJ, Berkhof J. Safety of extending screening intervals beyond five
years in cervical screening programmes with testing for high risk human papillomavirus:
14year follow-up of population based randomised cohort in the Netherlands. BMJ. 2016;
355: i4924.
invasive cervical cancer in Guadalajara, Mexico? Int J Cancer. 1999; 82(6): 804-809.
64) Crocetti E, Battisti L, Betta A, Palma PD, Paci E, Piffer S, Pojer A, Polla E, Zappa
M. The cytological screening turned out effective also for adenocarcinoma: a populationbased case-control study in Trento, Italy. Eur J Cancer Prev. 2007; 16(6): 564-567.
65) Kasinpila C, Promthet S, Vatanasapt P, Sasieni P, Parkin DM. Evaluation of the
nationwide cervical screening programme in Thailand: a case-control study. J Med
Screen. 2011; 18(3): 147-153.
66) Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, Cheung LC,
Raine-Bennett TR, Gage JC, Kinney WK. Benchmarking CIN 3+ risk as the basis for
incorporating HPV and Pap cotesting into cervical screening and management
guidelines. J Low Genit Tract Dis. 2013; 17(5 Suppl 1): S28-35.
67) Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Robertson M, Castle PE; New Mexico
HPV Pap Registry Steering Committee. The influence of type-specific human
papillomavirus infections on the detection of cervical precancer and cancer: a populationbased study of opportunistic cervical screening in the United States. Int J Cancer. 2014;
135(3): 624-634.
68) Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, de Sanjose S,
Naucler P, Lloveras B, Kjaer S, Cuzick J, van Ballegooijen M, Clavel C, Iftner T. Long
term predictive values of cytology and human papillomavirus testing in cervical cancer
screening: joint European cohort study. BMJ. 2008; 337: a1754.
69) Arbyn M, Roelens J, Simoens C, Buntinx F, Paraskevaidis E, Martin-Hirsch PP,
Prendiville WJ. Human papillomavirus testing versus repeat cytology for triage of minor
cytological cervical lesions. Cochrane Database Syst Rev. 2013; 3: CD008054.
70) Elfström KM, Smelov V, Johansson AL, Eklund C, Nauclér P, Arnheim-Dahlström L,
Dillner J. Long term duration of protective effect for HPV negative women: follow-up of
primary HPV screening randomised controlled trial. BMJ. 2014; 348: g130.
71) Smelov V, Elfström KM, Johansson AL, Eklund C, Naucler P, Arnheim-Dahlström L,
Dillner J. Long-term HPV type-specific risks of high-grade cervical intraepithelial
lesions: a 14-year follow-up of a randomized primary HPV screening trial. Int J Cancer.
2015; 136(5): 1171-1180.
72) Dijkstra MG, van Zummeren M, Rozendaal L, van Kemenade FJ, Helmerhorst TJ,
Snijders PJ, Meijer CJ, Berkhof J. Safety of extending screening intervals beyond five
years in cervical screening programmes with testing for high risk human papillomavirus:
14year follow-up of population based randomised cohort in the Netherlands. BMJ. 2016;
355: i4924.