よむ、つかう、まなぶ。
参考資料4 有効性評価に基づく子宮頸がん検診ガイドライン更新版2020年3月31日 (66 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_25869.html |
| 出典情報 | がん検診のあり方に関する検討会(第35回 5/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
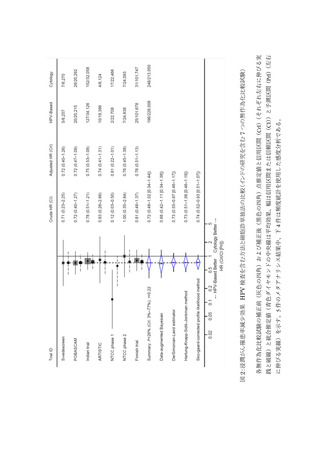
73) Uijterwaal MH, Polman NJ, Van Kemenade FJ, Van Den Haselkamp S, Witte BI,
Rijkaart D, Berkhof J, Snijders PJ, Meijer CJ. Five-Year Cervical (Pre) Cancer Risk of
Women Screened by HPV and Cytology Testing. Cancer Prev Res. 2015; 8(6): 502-508.
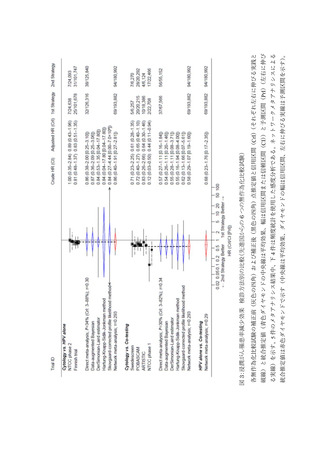
74) Luyten A, Buttmann-Schweiger N, Luyten K, Mauritz C, Reinecke-Lüthge A,
Pietralla M, Meijer CJ, Petry KU. Early detection of CIN3 and cervical cancer during
long-term follow-up using HPV/Pap smear co-testing and risk-adapted follow-up in a
locally organised screening programme. Int J Cancer. 2014; 135(6): 1408-1416.
75) Schiffman M, Glass AG, Wentzensen N, Rush BB, Castle PE, Scott DR, Buckland J,
Sherman ME, Rydzak G, Kirk P, Lorincz AT, Wacholder S, Burk RD. A long-term
prospective study of type-specific human papillomavirus infection and risk of cervical
neoplasia among 20,000 women in the Portland Kaiser Cohort Study. Cancer Epidemiol
Biomarkers Prev. 2011; 20(7): 1398-1409.
76) Chen HC, Schiffman M, Lin CY, Pan MH, You SL, Chuang LC, Hsieh CY, Liaw KL,
Hsing AW, Chen CJ; CBCSP-HPV Study Group. Persistence of type-specific human
papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer
Inst. 2011; 103(18): 1387-1396.
77) Hernández-Suárez G, Ortiz N, González M, Muñoz N. Short-term risk of cervical
intraepithelial neoplasia grades 2 and 3 for women with normal cytology and human
papillomavirus infection. Salud Publica Mex. 2010; 52(6): 486-492.
78) Mesher D, Szarewski A, Cadman L, Cubie H, Kitchener H, Luesley D, Menon U,
Hulman G, Desai M, Ho L, Terry G, Williams A, Sasieni P, Cuzick J. Long-term followup of cervical disease in women screened by cytology and HPV testing: results from the
HART study. Br J Cancer. 2010; 102(9): 1405-1410.
79) Cervical cancer screening(PDQ)―health professional version.
https://www.cancer.gov/types/cervical/hp/cervical-screening-pdq/ (アクセス;2018 年 10 月
1 日)
80) US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ,
Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld
CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB.
Screening for Cervical Cancer: US Preventive Services Task Force Recommendation
Statement. JAMA. 2018; 320(7): 674-686.
81) Canadian Task Force on Preventive Health Care. Recommendations on screening for
cervical cancer. CMAJ. 2013; 185(1): 35-45.
82) The UK NSC recommendation on cervical cancer screening in women.
https://legacyscreening.phe.org.uk/cervicalcancer. (アクセス;2018 年 10 月 1 日)
Rijkaart D, Berkhof J, Snijders PJ, Meijer CJ. Five-Year Cervical (Pre) Cancer Risk of
Women Screened by HPV and Cytology Testing. Cancer Prev Res. 2015; 8(6): 502-508.
74) Luyten A, Buttmann-Schweiger N, Luyten K, Mauritz C, Reinecke-Lüthge A,
Pietralla M, Meijer CJ, Petry KU. Early detection of CIN3 and cervical cancer during
long-term follow-up using HPV/Pap smear co-testing and risk-adapted follow-up in a
locally organised screening programme. Int J Cancer. 2014; 135(6): 1408-1416.
75) Schiffman M, Glass AG, Wentzensen N, Rush BB, Castle PE, Scott DR, Buckland J,
Sherman ME, Rydzak G, Kirk P, Lorincz AT, Wacholder S, Burk RD. A long-term
prospective study of type-specific human papillomavirus infection and risk of cervical
neoplasia among 20,000 women in the Portland Kaiser Cohort Study. Cancer Epidemiol
Biomarkers Prev. 2011; 20(7): 1398-1409.
76) Chen HC, Schiffman M, Lin CY, Pan MH, You SL, Chuang LC, Hsieh CY, Liaw KL,
Hsing AW, Chen CJ; CBCSP-HPV Study Group. Persistence of type-specific human
papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer
Inst. 2011; 103(18): 1387-1396.
77) Hernández-Suárez G, Ortiz N, González M, Muñoz N. Short-term risk of cervical
intraepithelial neoplasia grades 2 and 3 for women with normal cytology and human
papillomavirus infection. Salud Publica Mex. 2010; 52(6): 486-492.
78) Mesher D, Szarewski A, Cadman L, Cubie H, Kitchener H, Luesley D, Menon U,
Hulman G, Desai M, Ho L, Terry G, Williams A, Sasieni P, Cuzick J. Long-term followup of cervical disease in women screened by cytology and HPV testing: results from the
HART study. Br J Cancer. 2010; 102(9): 1405-1410.
79) Cervical cancer screening(PDQ)―health professional version.
https://www.cancer.gov/types/cervical/hp/cervical-screening-pdq/ (アクセス;2018 年 10 月
1 日)
80) US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ,
Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld
CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB.
Screening for Cervical Cancer: US Preventive Services Task Force Recommendation
Statement. JAMA. 2018; 320(7): 674-686.
81) Canadian Task Force on Preventive Health Care. Recommendations on screening for
cervical cancer. CMAJ. 2013; 185(1): 35-45.
82) The UK NSC recommendation on cervical cancer screening in women.
https://legacyscreening.phe.org.uk/cervicalcancer. (アクセス;2018 年 10 月 1 日)