【資料No.1】2.5_臨床に関する概括資料 (100 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26901.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第3回 7/20)、医薬品第二部会(令和4年度第6回 7/20)(合同開催)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
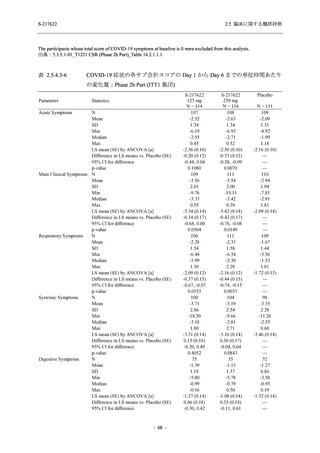
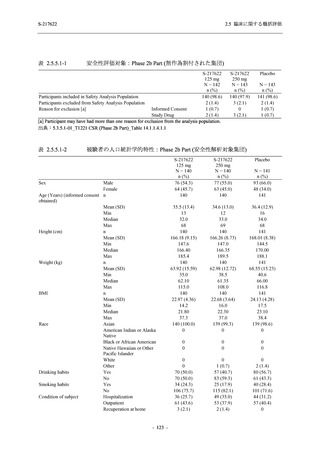
2.5 臨床に関する概括評価
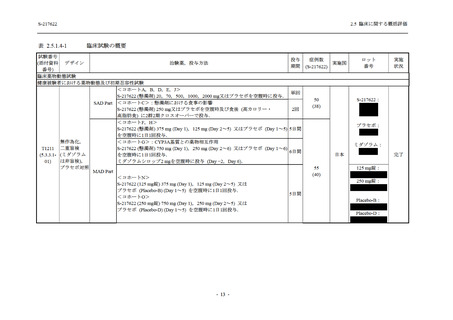
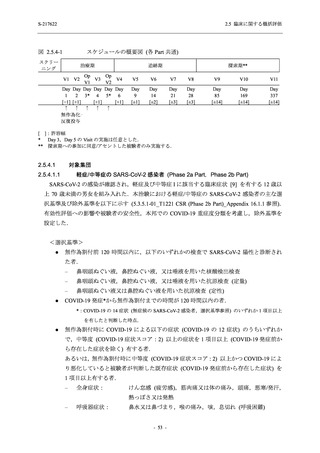
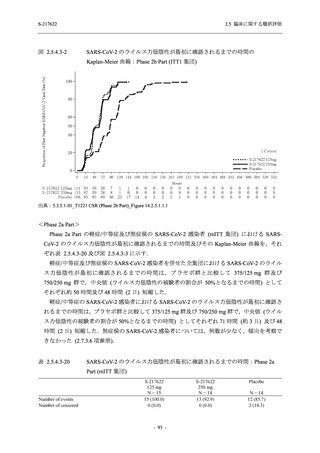
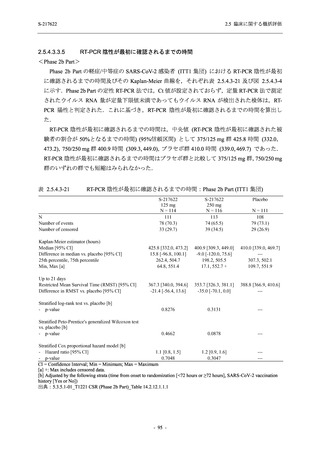
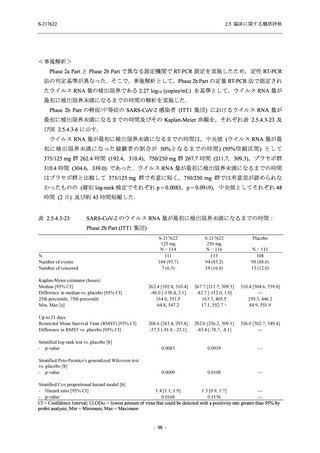
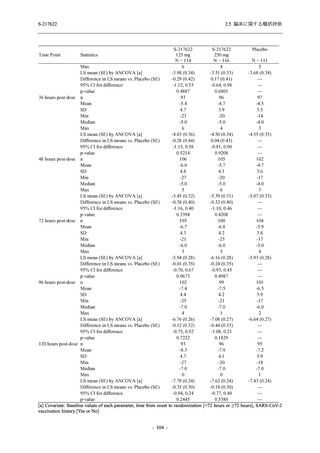
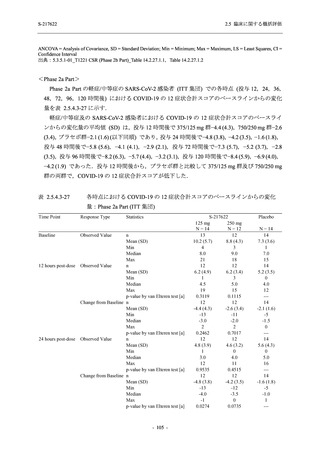
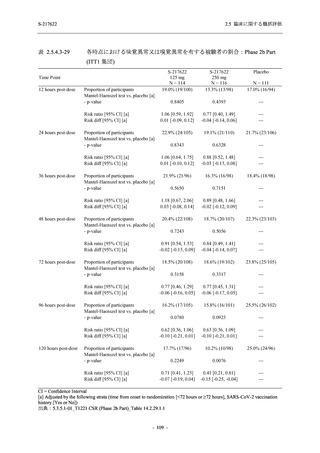
S-217622
125 mg
N = 114
S-217622
250 mg
N = 116
Placebo
N = 111
Kaplan-Meier estimator (hours)
Median [95% CI]
Difference in median vs. placebo [95% CI]
25th percentile, 75th percentile
Min, Max [a]
28.0 [21.5, 36.6]
-8.6 [-16.9, 5.0]
14.7, 59.7
0.2, 442.0
27.8 [24.6, 40.0]
-8.7 [-15.3, 10.3]
15.8, 66.1
0.2, 240.2
36.6 [28.0, 40.8]
--14.0, 63.2
0.5, 471.0 +
Up to 21 days
Restricted Mean Survival Time (RMST) [95% CI]
Difference in RMST vs. placebo [95% CI]
47.3 [35.7, 58.9]
-1.7 [-18.4, 14.9]
46.6 [37.9, 55.4]
-2.4 [-17.2, 12.3]
49.1 [37.2, 61.0]
---
Stratified log-rank test vs. placebo [b]
- p-value
0.7956
0.9331
---
Stratified Peto-Prentice's generalized Wilcoxon test
vs. placebo [b]
- p-value
0.5070
0.8432
---
Stratified Cox proportional hazard model [b]
- Hazard ratio [95% CI]
1.0 [0.8, 1.4]
1.0 [0.8, 1.3]
--- p-value
0.8233
0.9651
--CI = Confidence Interval; Min = Minimum; Max = Maximum
COVID-19 symptoms were 12 symptoms (low energy or tiredness, muscle or body aches, headache, chills or shivering,
feeling hot or feverish, stuffy or runny nose, sore throat, cough, shortness of breath [difficulty breathing], nausea, vomiting,
and diarrhea)
[a] +: Max includes censored data.
[b] Adjusted by the following strata (time from onset to randomization [<72 hours or ≥72 hours], SARS-CoV-2 vaccination
history [Yes or No])
出典:5.3.5.1-01_T1221 CSR (Phase 2b Part)_Table 14.2.23.1.1
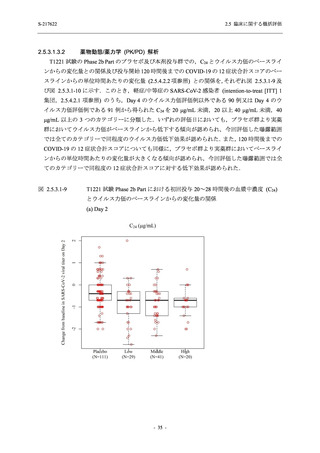
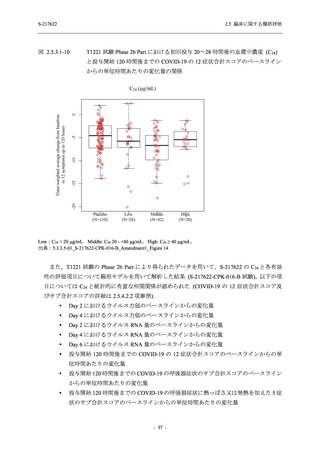
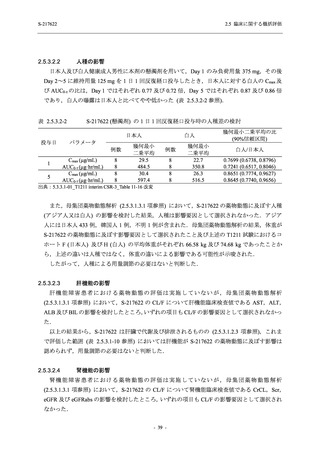
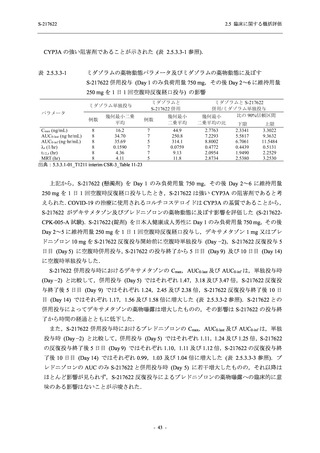
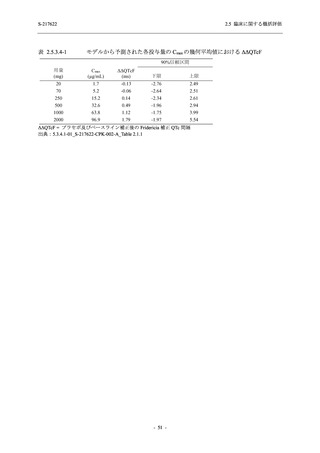
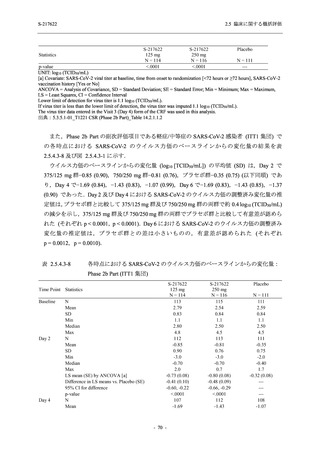
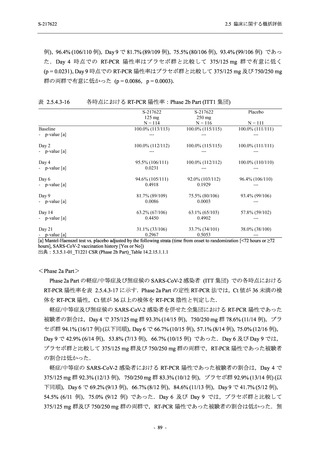
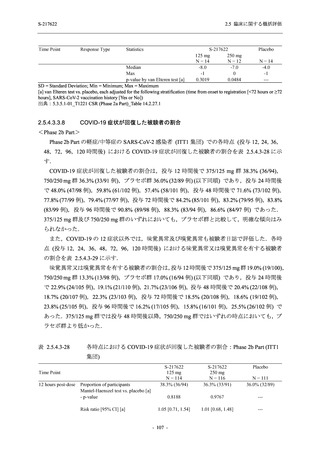
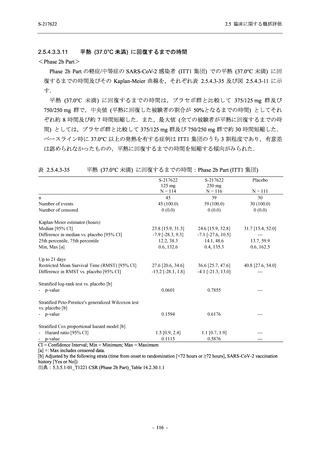
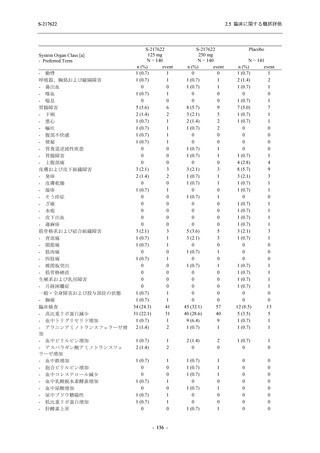
図 2.5.4.3-7
COVID-19 症状が回復するまでの時間の Kaplan-Meier 曲線:Phase 2b Part
(ITT1 集団)
出典:5.3.5.1-01_T1221 CSR (Phase 2b Part)_Figure 14.2.23.1.1
- 100 -