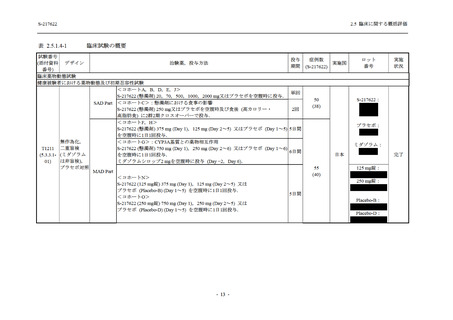
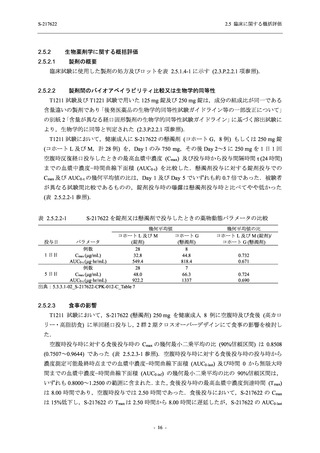
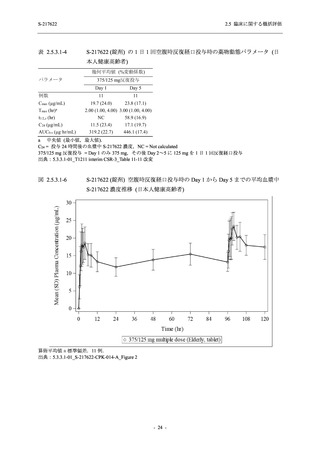
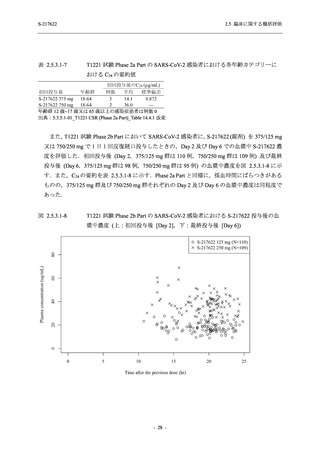
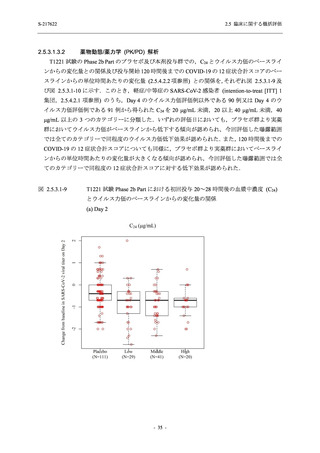
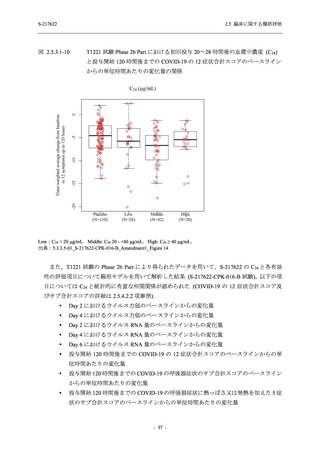
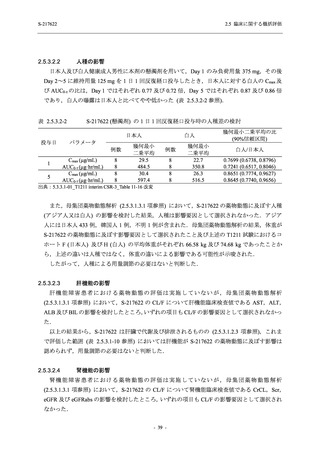
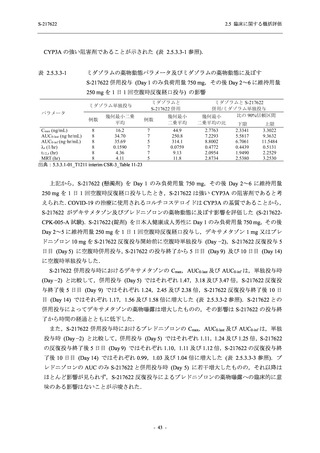
【資料No.1】2.5_臨床に関する概括資料 (71 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26901.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第3回 7/20)、医薬品第二部会(令和4年度第6回 7/20)(合同開催)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
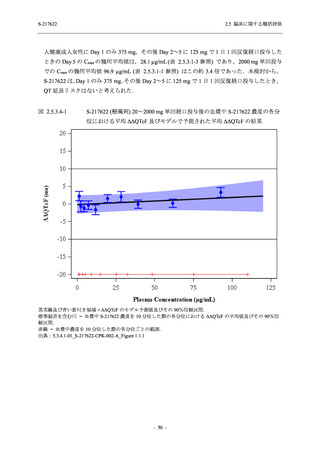
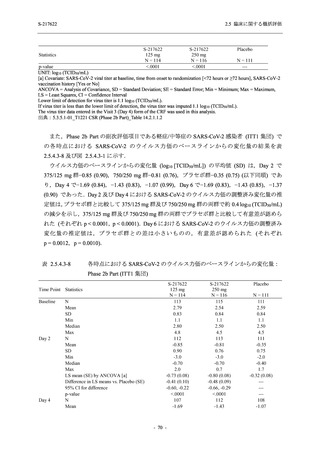
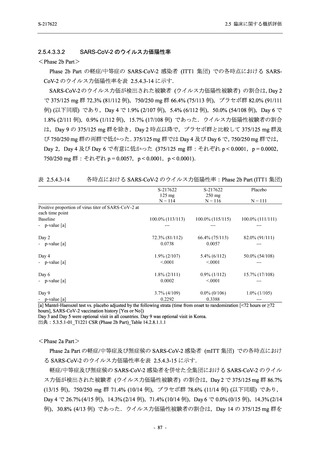
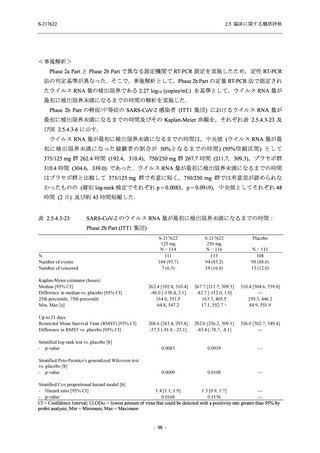
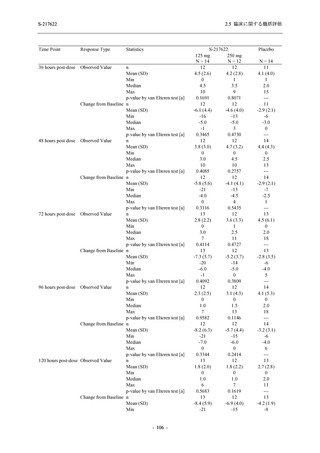
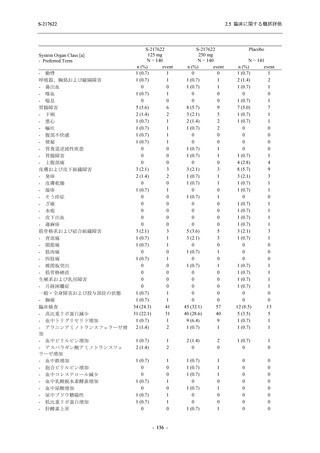
Time Point Statistics
2.5 臨床に関する概括評価
S-217622
125 mg
N = 114
0.84
-3.7
-1.70
0.0
-1.49 (0.04)
-0.41 (0.05)
-0.51, -0.31
<.0001
111
-1.69
0.83
-3.7
-1.70
0.0
-1.51 (0.02)
-0.10 (0.03)
-0.15, -0.04
0.0012
109
-1.69
0.83
-3.7
-1.70
0.0
-1.54 (0.01)
0.01 (0.01)
0.00, 0.03
0.1297
S-217622
250 mg
N = 116
0.83
-3.4
-1.40
0.0
-1.49 (0.04)
-0.41 (0.05)
-0.50, -0.31
<.0001
112
-1.43
0.85
-3.4
-1.40
0.0
-1.51 (0.02)
-0.10 (0.03)
-0.15, -0.04
0.0010
106
-1.46
0.86
-3.4
-1.40
0.0
-1.55 (0.01)
0.00 (0.01)
-0.02, 0.02
0.9932
Placebo
N = 111
SD
0.99
Min
-3.0
Median
-1.00
Max
2.0
LS mean (SE) by ANCOVA [a]
-1.08 (0.04)
Difference in LS means vs. Placebo (SE)
--95% CI for difference
--p-value
--Day 6
N
108
Mean
-1.37
SD
0.90
Min
-3.4
Median
-1.00
Max
1.4
LS mean (SE) by ANCOVA [a]
-1.42 (0.02)
Difference in LS means vs. Placebo (SE)
--95% CI for difference
--p-value
--Day 9
N
105
Mean
-1.48
SD
0.83
Min
-3.4
Median
-1.40
Max
0.0
LS mean (SE) by ANCOVA [a]
-1.55 (0.01)
Difference in LS means vs. Placebo (SE)
--95% CI for difference
--p-value
--UNIT: log10 (TCID50/mL)
[a] Covariate: SARS-CoV-2 viral titer at baseline, time from onset to randomization [<72 hours or ≥72 hours], SARS-CoV-2
vaccination history [Yes or No]
ANCOVA = Analysis of Covariance, SD = Standard Deviation; SE = Standard Error; Min = Minimum; Max = Maximum,
LS = Least Squares, CI = Confidence Interval
Day 3 and Day 5 were optional visit in all countries. Day 9 was optional visit in Korea.
Lower limit of detection for virus titer is 1.1 log10 (TCID50/mL). If virus titer is less than the lower limit of detection, the
virus titer was imputed 1.1 log10 (TCID50/mL).
The virus data at the analysis visit specified within the acceptable time window was used in this analysis.
出典:5.3.5.1-01_T1221 CSR (Phase 2b Part)_Table 14.2.1.1.7,Table 14.2.1.1.10
- 71 -