【資料No.1】2.5_臨床に関する概括資料 (30 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26901.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第3回 7/20)、医薬品第二部会(令和4年度第6回 7/20)(合同開催)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
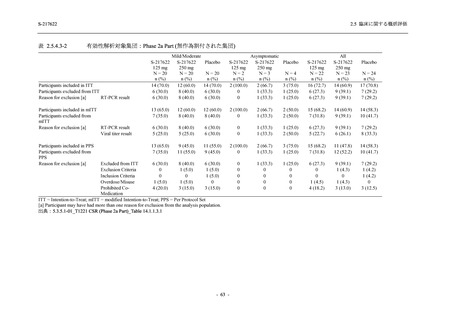
2.5 臨床に関する概括評価
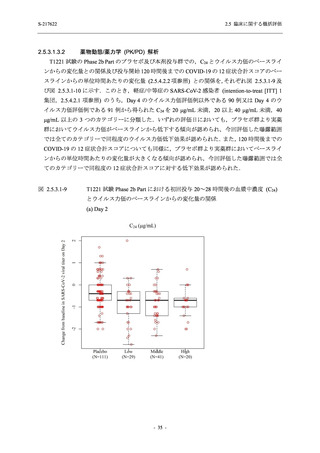
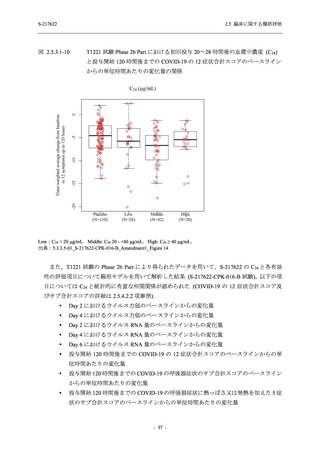
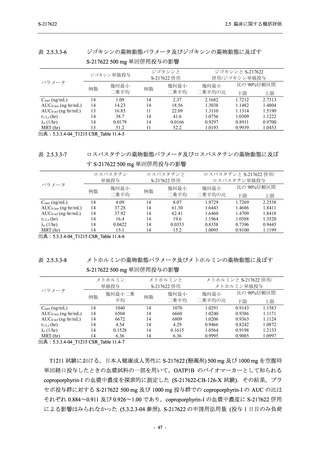
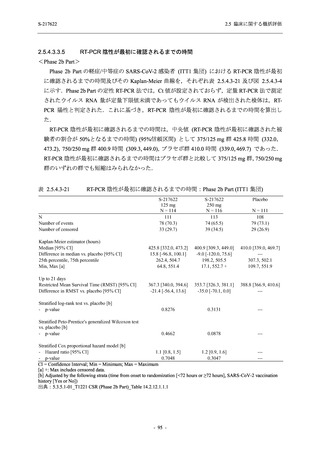
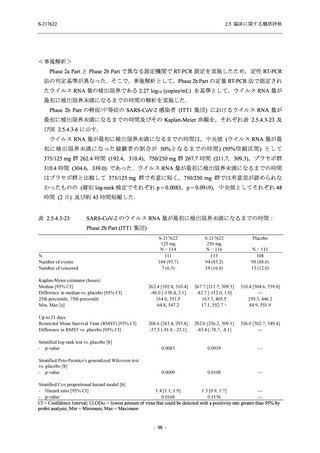
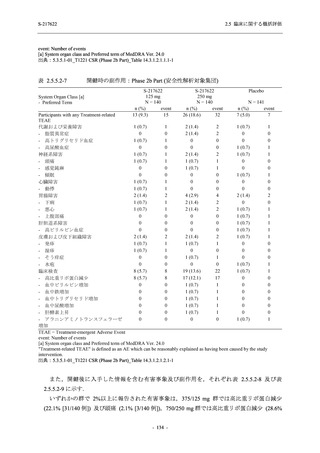
表 2.5.3.1-9
Study title
A Phase 1
Study of
S-217622
(T1211)
母集団薬物動態解析に用いた試験の概要
Cohort/
part
A, B, D,
E, and J
Dosing regimen
PK sampling time
A single dose of S-217622 (suspension) 20,
70, 500, 1000, and 2000 mg in the fasted
state in Japanese healthy adult male
participants.
Cohort A:
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, 24, 36, 48, 60, 72, 96, 120, and
144 hours postdose on Day 1
C
A single dose of S-217622 (suspension)
250 mg in the fasted state or in the fed state
(high-fat, high-calorie) in Japanese healthy
adult male participants.
F, H
Multiple doses of S-217622 (suspension)
375 mg on Day 1 and 125 mg once daily on
Days 2 to 5 in the fasted state in Japanese
and White healthy adult male participants.
Multiple doses of S-217622 (suspension)
750 mg on Day 1 and 250 mg once daily on
Days 2 to 6 in the fasted state in Japanese
healthy adult male participants.
Multiple doses of S-217622 (tablet) 750 mg
on Day 1 and 250 mg once daily on Days 2
to 5 in the fasted state in Japanese healthy
adult male participants.
Cohort B, D, E, and J:
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, 24, 36, 48, 60, 72, 96, 120, 144, 192,
and 336 hours postdose on Day 1
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, 24, 36, 48, 60, 72, 96, 120, 144, and
192 hours postdose on Day 1 and predose
(0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12,
24, 36, 48, 60, 72, 96, 120, 144, 192, and
336 hours postdose on Day 15
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, and 12 hours postdose on Day 1
G
L, M
N, O
P
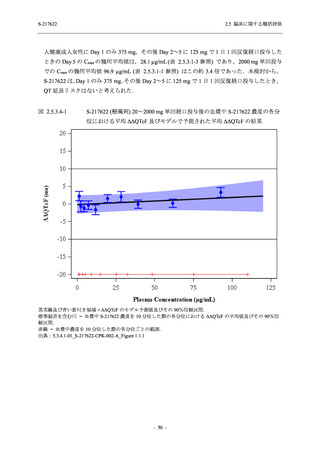
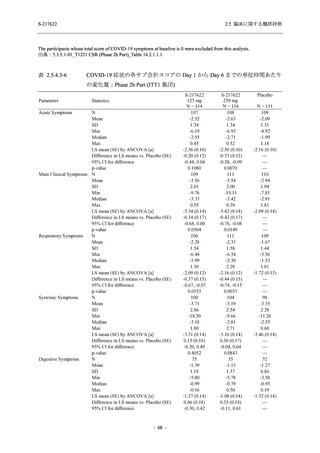
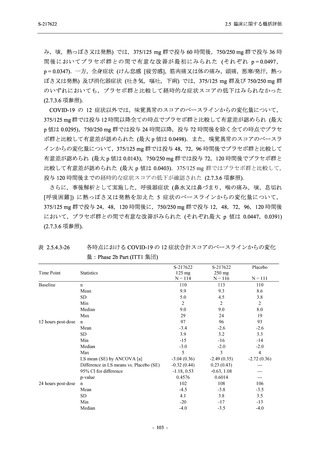
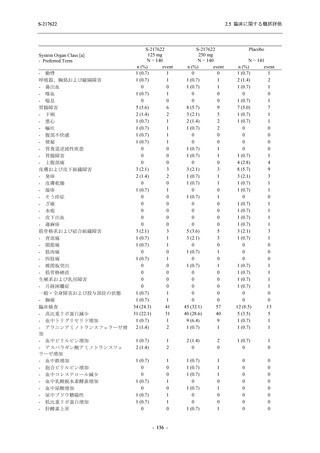
A Phase 2/3
Study of
S-217622
(T1221)
Phase 2a
Part and
Phase 2b
Part
Multiple doses of S-217622 (tablet) 375 mg
on Day 1 and 125 mg once daily on Days 2
to 5 or 750 mg on Day 1 and 250 mg once
daily on Days 2 to 5 in the fasted state in
Japanese healthy adult female participants.
A single dose of S-217622 (tablet) 375 mg
in the fasted state or in the fed state
(high-fat, high-calorie) in Japanese healthy
adult participants.
High dose group (750/250 mg group):
Multiple doses of S-217622 750 mg on
Day 1 and 250 mg on Days 2 to 5 once
daily in Asian (Japanese and Korean)
participants infected with SARS-CoV-2.
Low dose group (375/125 mg group):
Multiple doses of S-217622 375 mg on
Day 1 and 125 mg on Days 2 to 5 once
daily in Asian (Japanese and Korean)
participants infected with SARS-CoV-2.
- 30 -
Predose (0 hours) on Day 2, Day 3, and
Day 4
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, and 24 hours postdose on Day 5
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, and 12 hours postdose on Day 1
Predose (0 hours) on Day 2
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, 24, 96, 120, 216, 240, and 312 hours
postdose on Day 5
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, and 12 hours postdose on Day 1
Predose (0 hours) on Day 2, Day 3, and
Day 4
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, 24, 48, 72, and 96 hours postdose on
Day 5 and on Day 13 (at visit)
Predose (0 hours), 0.5, 1, 1.5, 2, 2.5, 3, 4, 6,
8, 12, 16, 24, 48, 72, 120, 168, and
336 hours postdose on Day 1 and Day 22
On Day 2 and Day 6