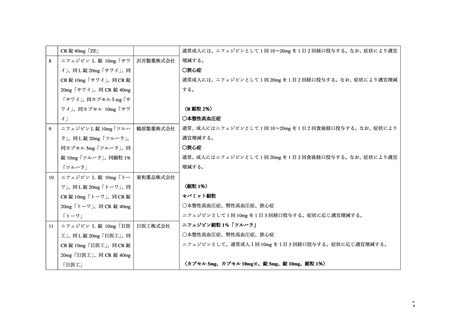
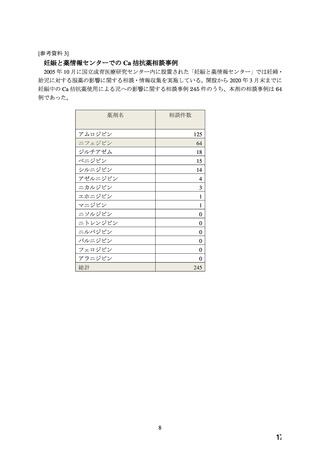
よむ、つかう、まなぶ。
資料1-3 ニフェジピン 調査結果報告書及び添付文書 (21 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
hypertension can provide substantial benefit. Relative risk reduction from blood pressure
reduction is similar across populations with varying absolute risk, so the absolute benefit is
greater in patients who are at higher risk independent of their hypertension (for example, patients
with diabetes or hyperlipidemia), and such patients would be expected to benefit from more
aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black
patients, and many antihypertensive drugs have additional approved indications and effects (e.g.,
on angina, heart failure, or diabetic kidney disease). These considerations may guide selection
of therapy.
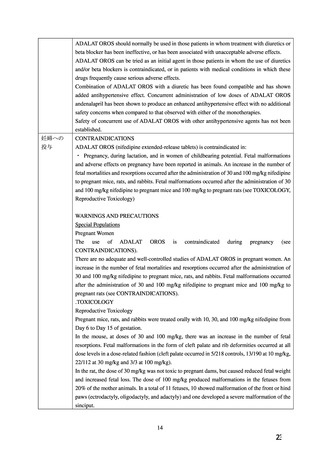
PROCARDIA XL may be used alone or in combination with other antihypertensive agents.
妊婦への
PRECAUTIONS
投与
Pregnancy:Pregnancy Category C:
Nifedipine has been shown to produce teratogenic findings in rats and rabbits, including digital
anomalies similar to those reported for phenytoin. Digital anomalies have been reported to
occur with other members of the dihydropyridine class and are possibly a result of
compromised uterine blood flow. Nifedipine administration was associated with a variety of
embryotoxic, placentotoxic, and fetotoxic effects, including stunted fetuses (rats, mice,
rabbits), rib deformities (mice), cleft palate (mice), small placentas and underdeveloped
chorionic villi (monkeys), embryonic and fetal deaths (rats, mice, rabbits), and prolonged
pregnancy/decreased neonatal survival (rats; not evaluated in other species). On a mg/kg basis,
all of the doses associated with the teratogenic embryotoxic or fetotoxic effects in animals
were higher (5 to 50 times) than the maximum recommended human dose of 120 mg/day. On a
mg/m2 basis, some doses were higher and some were lower than the maximum recommended
human dose but all were within an order of magnitude of it. The doses associated with
placentotoxic effects in monkeys were equivalent to or lower than the maximum recommended
human dose on a mg/m2 basis.
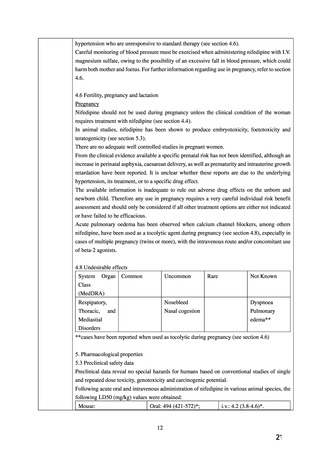
There are no adequate and well-controlled studies in pregnant women. PROCARDIA should be
used during pregnancy only if the potential benefit justifies the potential
経口剤(英国)
(2) 製品名 Adalat LA 30 mg prolonged-release tablets/ Bayer plc
効能・効果
4. Clinical particulars
4.1 Therapeutic indications
For the treatment of all grades of hypertension.
For the prophylaxis of chronic stable angina pectoris either as monotherapy or in combination
with a beta-blocker.
妊婦への
4. Clinical particulars
投与
4.4 Special warnings and precautions for use
Adalat LA should not be used during pregnancy unless the clinical condition of the woman
requires treatment with nifedipine. Adalat LA should be reserved for women with severe
11
20
reduction is similar across populations with varying absolute risk, so the absolute benefit is
greater in patients who are at higher risk independent of their hypertension (for example, patients
with diabetes or hyperlipidemia), and such patients would be expected to benefit from more
aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black
patients, and many antihypertensive drugs have additional approved indications and effects (e.g.,
on angina, heart failure, or diabetic kidney disease). These considerations may guide selection
of therapy.
PROCARDIA XL may be used alone or in combination with other antihypertensive agents.
妊婦への
PRECAUTIONS
投与
Pregnancy:Pregnancy Category C:
Nifedipine has been shown to produce teratogenic findings in rats and rabbits, including digital
anomalies similar to those reported for phenytoin. Digital anomalies have been reported to
occur with other members of the dihydropyridine class and are possibly a result of
compromised uterine blood flow. Nifedipine administration was associated with a variety of
embryotoxic, placentotoxic, and fetotoxic effects, including stunted fetuses (rats, mice,
rabbits), rib deformities (mice), cleft palate (mice), small placentas and underdeveloped
chorionic villi (monkeys), embryonic and fetal deaths (rats, mice, rabbits), and prolonged
pregnancy/decreased neonatal survival (rats; not evaluated in other species). On a mg/kg basis,
all of the doses associated with the teratogenic embryotoxic or fetotoxic effects in animals
were higher (5 to 50 times) than the maximum recommended human dose of 120 mg/day. On a
mg/m2 basis, some doses were higher and some were lower than the maximum recommended
human dose but all were within an order of magnitude of it. The doses associated with
placentotoxic effects in monkeys were equivalent to or lower than the maximum recommended
human dose on a mg/m2 basis.
There are no adequate and well-controlled studies in pregnant women. PROCARDIA should be
used during pregnancy only if the potential benefit justifies the potential
経口剤(英国)
(2) 製品名 Adalat LA 30 mg prolonged-release tablets/ Bayer plc
効能・効果
4. Clinical particulars
4.1 Therapeutic indications
For the treatment of all grades of hypertension.
For the prophylaxis of chronic stable angina pectoris either as monotherapy or in combination
with a beta-blocker.
妊婦への
4. Clinical particulars
投与
4.4 Special warnings and precautions for use
Adalat LA should not be used during pregnancy unless the clinical condition of the woman
requires treatment with nifedipine. Adalat LA should be reserved for women with severe
11
20