よむ、つかう、まなぶ。
資料1-3 ニフェジピン 調査結果報告書及び添付文書 (47 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
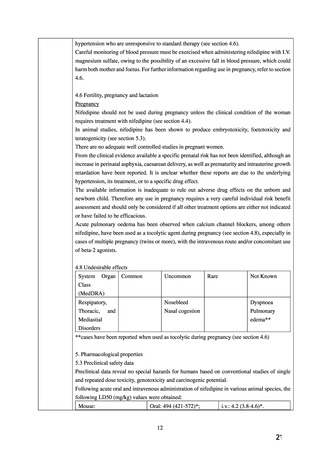
8.9.1 Hypertension and pregnancy
Women with pre-existing hypertension may continue their current antihypertensive medication, but ACE
inhibitors, ARBs, and direct renin inhibitors are contraindicated due to adverse foetal and neonatal
outcomes. Methyldopa, labetalol, and CCBs are the drugs of choice.
海外ガイドライン
英国関連学会
National Collaborating Centre for Women’s and Children’s Health, Royal College of
Obstetricians and Gynaecologists, British and Irish Hypertension Society
(10) Hypertension in pregnancy: diagnosis and management (2019) NICE guideline CG107. National
Institute for Health and Care Excellence
1.3 Management of pregnancy with chronic hypertension
Pre-pregnancy advice
1.3.1 Offer women with chronic hypertension referral to a specialist in hypertensive disorders of pregnancy
to discuss the risks and benefits of treatment. [2010, amended 2019]
1.3.2 Advise women who take angiotensin-converting enzyme (ACE) inhibitors and angiotensin II
receptor blockers (ARBs):
・ that there is an increased risk of congenital abnormalities if these drugs are taken during pregnancy
・ to discuss other antihypertensive treatment with the healthcare professional responsible for
managing their hypertension, if they are planning pregnancy.
・to discuss alternative treatment with the healthcare professional responsible for managing their
condition, if ACE inhibitors or ARBs are being taken for other conditions such as renal disease.
[2010, amended 2019]
Treatment of chronic hypertension
1.3.10 Consider labetalol to treat chronic hypertension in pregnant women. Consider nifedipine[3] for
women in whom labetalol is not suitable, or methyldopa if both labetalol and nifedipine[3] are not
suitable. Base the choice on any pre-existing treatment, side-effect profiles, risks (including fetal
effects) and the woman's preference. [2019]
[3] At the time of publication (June 2019), some brands of nifedipine were specifically contraindicated
during pregnancy by the manufacturer in its summary of product characteristics. Refer to the
individual summaries of product characteristics for each preparation of nifedipine for further details.
海外のガイドライン カナダ産科婦人科学会・カナダ高血圧学会
(11) Butalia Set al, Hypertension Canada's 2018 Guidelines for the Management of Hypertension in
Pregnancy. Can J Cardiol. 2018 May;34(5):526-531. doi: 10.1016/j.cjca.2018.02.021. Epub 2018 Mar
1. PMID: 29731014
Hypertension Canada’s 2018 Guidelines: Management of Hypertension in Pregnancy
I.
Management of nonsevere hypertension (BP 140-159/90-109 mm Hg) in pregnancy
Guidelines
2. A. Initial antihypertensive therapy should be monotherapy from the following first-line drugs:
oral labetalol, oral methyldopa, long-acting oral nifedipine, or other oral b-blockers (acebutolol,
metoprolol, pindolol, and propranolol) (Grade C).
37
46
Women with pre-existing hypertension may continue their current antihypertensive medication, but ACE
inhibitors, ARBs, and direct renin inhibitors are contraindicated due to adverse foetal and neonatal
outcomes. Methyldopa, labetalol, and CCBs are the drugs of choice.
海外ガイドライン
英国関連学会
National Collaborating Centre for Women’s and Children’s Health, Royal College of
Obstetricians and Gynaecologists, British and Irish Hypertension Society
(10) Hypertension in pregnancy: diagnosis and management (2019) NICE guideline CG107. National
Institute for Health and Care Excellence
1.3 Management of pregnancy with chronic hypertension
Pre-pregnancy advice
1.3.1 Offer women with chronic hypertension referral to a specialist in hypertensive disorders of pregnancy
to discuss the risks and benefits of treatment. [2010, amended 2019]
1.3.2 Advise women who take angiotensin-converting enzyme (ACE) inhibitors and angiotensin II
receptor blockers (ARBs):
・ that there is an increased risk of congenital abnormalities if these drugs are taken during pregnancy
・ to discuss other antihypertensive treatment with the healthcare professional responsible for
managing their hypertension, if they are planning pregnancy.
・to discuss alternative treatment with the healthcare professional responsible for managing their
condition, if ACE inhibitors or ARBs are being taken for other conditions such as renal disease.
[2010, amended 2019]
Treatment of chronic hypertension
1.3.10 Consider labetalol to treat chronic hypertension in pregnant women. Consider nifedipine[3] for
women in whom labetalol is not suitable, or methyldopa if both labetalol and nifedipine[3] are not
suitable. Base the choice on any pre-existing treatment, side-effect profiles, risks (including fetal
effects) and the woman's preference. [2019]
[3] At the time of publication (June 2019), some brands of nifedipine were specifically contraindicated
during pregnancy by the manufacturer in its summary of product characteristics. Refer to the
individual summaries of product characteristics for each preparation of nifedipine for further details.
海外のガイドライン カナダ産科婦人科学会・カナダ高血圧学会
(11) Butalia Set al, Hypertension Canada's 2018 Guidelines for the Management of Hypertension in
Pregnancy. Can J Cardiol. 2018 May;34(5):526-531. doi: 10.1016/j.cjca.2018.02.021. Epub 2018 Mar
1. PMID: 29731014
Hypertension Canada’s 2018 Guidelines: Management of Hypertension in Pregnancy
I.
Management of nonsevere hypertension (BP 140-159/90-109 mm Hg) in pregnancy
Guidelines
2. A. Initial antihypertensive therapy should be monotherapy from the following first-line drugs:
oral labetalol, oral methyldopa, long-acting oral nifedipine, or other oral b-blockers (acebutolol,
metoprolol, pindolol, and propranolol) (Grade C).
37
46